Abstract
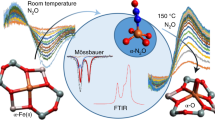
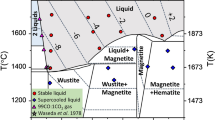
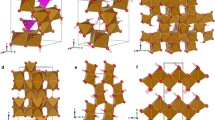
WE have published results1 of a study of the kinetics of the oxidation of a sample of Fe3O4 consisting of submicron crystals almost perfectly cubic and almost uniform in size2. In agreement with Egger and W. F.3 and W. F.4,5, it was found that the reaction was single phase topotactic (Fe3O4→Fe3−xO4→γ-Fe2O3). The mechanism proposed was based on the diffusion of iron cations, the coefficient of which was found to depend on the Fe2+/total iron ratio. When a model of this mechanism was simulated on a computer, an accurate prediction was obtained of the kinetic behaviour over all composition ranges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gallagher, K. J., Feitknecht, W., and Mannweiler, U., Nature, 217, 1118 (1968).
Mannweiler, U., Chimia, 20, 363 (1966).
Egger, K., and Feitknecht, W., Helv. Chim. Acta, 45, 2042 (1962).
Feitknecht, W., CR Coll. Int. du CNRS, No. 122, 121 (Paris, 1963).
Feitknecht, W., Pure Appl. Chem., 9, 423 (1964).
Colombo, U., Fagherazzi, G., Gazzarini, F., Lanzavecchia, G., and Sironi, G., Nature, 219, 1036 (1968).
Mannweiler, U., thesis, Univ. Bern (1965).
Feitknecht, W., and Mannweiler, U., Helv. Chim. Acta, 50, 570 (1967).
Colombo, U., Gazzarini, F., and Lanzavecchia, G., Mat. Sci. Eng., 2, 125 (1967).
Gazzarini, F., and Lanzavecchia, G., Reactivity of Solids, Proc. Sixth I.S.R.S., p. 57 (Wiley-Interscience, 1969).
Fricke, R., Z. Elektrochem. Angew. Phys. Chem., 46, 491 (1940).
Wagner, C. N. J., Advances in X-Ray Anal., 7, 47 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FEITKNECHT, W., GALLAGHER, K. Mechanisms for the Oxidation of Fe3O4. Nature 228, 548–549 (1970). https://doi.org/10.1038/228548a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/228548a0
This article is cited by
-
Structural, morphology, optical, and magnetic study of room-temperature ferromagnetic α-Fe2O3 nanofibers
Applied Physics A (2023)
-
Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions
Nature Communications (2022)
-
Preparation of High-purity 1,3-Diacylglycerol Using Performance-enhanced Lipase Immobilized on Nanosized Magnetite Particles
Biotechnology and Bioprocess Engineering (2019)
-
Enhanced Performance of Rhizopus oryzae Lipase by Reasonable Immobilization on Magnetic Nanoparticles and Its Application in Synthesis 1,3-Diacyglycerol
Applied Biochemistry and Biotechnology (2019)
-
Size-dependent redox behavior of iron observed by in-situ single nanoparticle spectro-microscopy on well-defined model systems
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.