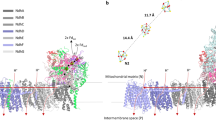
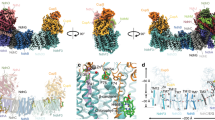
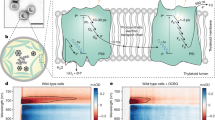
Abstract
AFTER the discovery of non-cyclic photosynthetic phosphorylation1, investigations in other laboratories2,3 confirmed the observation that one ATP molecule was formed for each pair of electrons (2e) transferred from water to TPN+ or ferricyanide. Later work with these electron acceptors4,5 and with quinones6 added support to the belief that the limit of the P : 2e ratio (μmoles ATP formed per two electrons transferred) is unity. Tris-HCl was used in this early work, and more recent studies using this buffer have confirmed the reported pH optimum of 7.8–8.0 and the P : 2e ratio of 1.0 (ref. 7). With the introduction of new buffers8 and the use of pH values between 8.6 and 8.9, overall P : 2e ratios greater than 1.0 have been observed7–9. Lynn and Brown10, using a different incubation procedure, claim to have obtained high P : 2e ratios with the tris buffer, and Shavit and Avron11 have obtained ratios greater than 1.0 with tris-HCl. On the other hand, Ramirez et al.12 have never-observed P : 2e ratios greater than 1.0 although the pH optima for ferricyanide and TPN+ reduction were 7.7 and 8.5, respectively, in the presence of tris, but were both 8.5 in the presence of ‘Tricine’ (N-tris (hydroxymethyl) methylglycine). The H+ : e (ratio of protons taken up to electrons transported; the value of this H+ : e ratio should equal the number of sites of energy conservation (ATP formation) in the electron transport path) varies strongly with pH showing a maximum value of 6 at pH 6.0 using diquat (N, N-ethylene-2,2-dipyridilium dibromide) as the electron acceptor; a similar variation with pH occurred in ferricyanide reduction with a maximum H+ : e = 4 (ref. 13). An H+ : e ratio of 5 was observed with chloranil as the electron acceptor9. Many different P : 2e and H+ : e ratios have thus been observed depending on the pH, the electron acceptor and the buffer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arnon, D. I., Whatley, F. R., and Allen, M. B., Science, 127, 1026 (1958).
Krogmann, D. W., Jagendorf, A. T., and Avron, M., Plant Physiol., 34, 272 (1959).
Good, N. E., Nature, 188, 661 (1960).
Stiller, M., and Vennesland, B., Biochim. Biophys. Acta, 60, 562 (1962).
Turner, J., Black, C. C., and Gibbs, M., J. Biol. Chem., 237, 577 (1962).
Trebst, A., and Eck, H., Z. Naturforsch., 166, 455 (1961).
Winget, G. D., Izawa, S., and Good, N. E., Biochem. Biophys. Res. Commun., 21, 438 (1965).
Good, N. E., Winget, G. D., Winter, W., Connolly, T. N., Izawa, S., and Singh, R. M. M., Biochemistry, 5, 467 (1966).
Izawa, S., Winget, G. D., and Good, N. E., Biochem. Biophys. Res. Commun., 22, 223 (1966).
Lynn, W. S., and Brown, R. H., J. Biol. Chem., 242, 412, 426 (1967).
Shavit, N., and Avron, M., Biochim. Biophys. Acta, 131, 516 (1967).
Ramirez, J. M., Del Campo, F. F., and Arnon, D. I., Fed. Proc. (Abstr.), 26, 861 (1967).
Karlish, S. J. D., and Avron, M., Nature, 126, 1107 (1967).
Arnon, D. I., Losada, M., Whatley, F. R., Tsujimoto, H. Y., Hall, D. O., and Horton, A. A., Proc. US Nat. Acad. Sci., 47, 1314 (1961).
Hagihara, B., and Lardy, H. A., J. Biol. Chem., 235, 889 (1960).
Hill, R., and Bendall, F., Nature, 187, 417 (1960).
Arnon, D. I., Plant Physiol., 24, 1 (1949).
Arnon, D. I., Tsujimoto, H. Y., and McSwain, B. D., Nature, 214, 562 (1967).
Avron, M., and Jagendorf, A. T., J. Biol. Chem., 234, 1315 (1959).
Arnon, D. I., in Biological Structure and Function, 2, 339 (edit. by Goodwin, T. W., and Lindberg, O.) (Academic Press, London and New York, 1961).
Jagendorf, A. T., and Margulies, M., Arch. Biochem. Biophys., 90, 184 (1960).
Vernon, L. P., and Zaugg, W. S., J. Biol. Chem., 235, 2728 (1960).
Petrack, B., and Lipmann, F., in Light and Life, 621 (edit. by McElroy, W. D., and Glass, B.) (Johns Hopkins Press, Baltimore, 1961).
Avron, M., J. Biol. Chem., 237, 2011 (1962).
Hoch, G., and Martin, I., Biochem. Biophys. Res. Commun., 12, 223 (1963).
Bennun, A., and Avron, M., Biochim. Biophys. Acta, 109, 117 (1965).
Carmeli, C., and Avron, M., European J. Biochem., 2, 318 (1967).
Shavit, N., Skye, G. E., and Boyer, P. D., J. Biol. Chem., 242, 5125 (1967).
Skye, G. E., Shavit, N., and Boyer, P. D., Biochem. Biophys. Res. Commun., 28, 724 (1967).
Fiske, C. H., and Subbarrow, Y., J. Biol. Chem., 66, 375 (1925).
Duysens, L. N. M., and Amesz, J., in Comprehensive Biochemistry (edit. by Florkin, M., and Stotz, E. H.), 27, 237 (Elsevier, Amsterdam, 1967).
Izawa, S., and Hind, G., Biochim. Biophys. Acta, 143, 377 (1967).
Jensen, R. G., and Bassham, J. A., Proc. US Nat. Acad. Sci., 56, 1095 (1966).
Kalberer, P. B., Buchanan, B. B., and Arnon, D. I., Proc. US Nat. Acad. Sci., 57, 1542 (1967).
Cockburn, W., Baldry, C. W., and Walker, D. A., Biochim. Biophys. Acta, 143, 606 (1967).
Avron, M., Krogmann, D. W., and Jagendorf, A. T., Biochim. Biophys. Acta, 30, 144 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HORTON, A., HALL, D. Determining the Stoichiometry of Photosynthetic Phosphorylation. Nature 218, 386–388 (1968). https://doi.org/10.1038/218386a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/218386a0
This article is cited by
-
Immobilization of thylakoids in porous particles and stabilization of the photochemical processes by glutaraldehyde action at subzero temperature
European Journal of Applied Microbiology and Biotechnology (1979)
-
Energy coupling in chloroplasts
Journal of Bioenergetics and Biomembranes (1976)
-
Nomenclature for Isolated Chloroplasts
Nature New Biology (1972)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.