Abstract
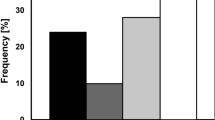
As a result of their high refractility and their resistance to stains and to lethal agents such as heat and chemicals, it has long been thought that bacterial spores are relatively dry and impermeable. There is conflicting evidence, however, about the degree of desiccation and impermeability which may exist. Refractive index measurements on spores in aqueous suspensions indicate very low water contents1, and the surface area of lyophilized spores available to adsorption of nitrogen gas is so small that little or no internal porosity can be postulated2. On the other hand, it has been demonstrated that spores have a significant affinity for water3. In aqueous media it is known that germinants enter spores rapidly and evidence has been presented for considerable porosity and a water content as high as 75 per cent of the dry weight4. In an effort to account for these discrepancies, we have employed standard gas adsorption techniques to measure water vapour sorption of intact and crushed spores and the nitrogen gas adsorption of spores which were dried in various ways. The results confirm the hydrophilic nature of spores and indicate that a considerable fraction of the volume occupied by water in wet spores can be preserved in the dry state by employing dehydration methods which minimize the collapse of hydrophilic, macromolecular structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ross, K. F. A., and Billing, E., J. Gen. Microbiol., 16, 418 (1957).
Berlin, E., Curran, H. R., and Pallansch, M. J., J. Bacteriol., 86, 1030 (1963).
Waldman, D. G., and Halvorson, H. O., Appl. Microbiol., 2, 333 (1954).
Black, S. H., and Gerhardt, P., J. Bacteriol., 83, 960 (1962).
Brunauer, S., Emmett, P. H., and Teller, E., J. Amer. Chem. Soc., 60, 309 (1938).
McLaren, A. D., and Rowen, J. W., J. Polymer Sci., 7, 289 (1951).
Stamm, A. J., Wood and Cellulose Science (Ronald Press Co., 1964).
Barkas, W. W., Trans. Faraday Soc., 38, 194 (1942).
Urquart, A. R., and Williams, A. M., J. Textile Inst., 28, T159 (1958).
Lewis, J. C., Snell, N. S., and Burr, H. K., Science, 132, 544 (1960).
Merchant, M. V., Tappi, 40, 771 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
NEIHOF, R., THOMPSON, J. & DEITZ, V. Sorption of Water Vapour and Nitrogen Gas by Bacterial Spores. Nature 216, 1304–1306 (1967). https://doi.org/10.1038/2161304a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/2161304a0
This article is cited by
-
Effect of the internal water activity of bacterial spores on their heat resistance in water
Current Microbiology (1984)
-
On the residual water content of dried but viable cells
Experientia (1978)
-
Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex
Nature (1975)
-
Porosity of swollen solvent-exchanged cellulose and its collapse during final liquid removal
Colloid and Polymer Science (1975)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.