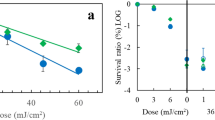
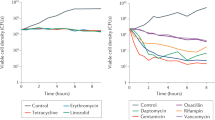
Abstract
THIOL-BINDING agents such as N-ethylmaleimide1, iodoacetamide2 and hydroxymercuribenzoate3 are effective bacterial radiosensitizers, particularly with the more radioresistant bacteria2. Sensitization of bacteria by these compounds is believed to be a result of their common ability to bind sulphydryl groups within the bacterial cell which can function either as natural radioprotective agents by radical scavenging or as repair agents for DNA radicals formed by hydrogen abstraction4–6. Alternatively, enzyme repair reactions may be inhibited by the presence of these sulphydryl poisons, a view recently emphasized by Alexander, Lett and Dean7. Another group of radiosensitizing compounds are the stable free radicals such as oxygen8, nitric oxide9, di-t-butyl nitroxide10, and copper ions11. Still a third class of compounds known to radiosensitize bacteria are halogen containing substances such as chloral hydrate12 or chloroform13 which presumably act by the formation of short-lived halogen radicals toxic to the bacterial cell (the halogen containing sulphydryl poison iodoacetamide may be in this category too) or by formation of long lived toxic radiolytic product. Most of these agents are found to be more effective in nitrogen than in oxygen radiation conditions. Halogenated pyrimidines such as 5-bromouracil sensitize by yet another mechanism, because they require incorporation into cellular DNA in order to be effective.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bridges, B. A., Nature, 188, 415 (1960).
Dean, C. J., and Alexander, P., Nature, 196, 1324 (1962).
Bruce, A. K., and Malchman, W. H., Radiat. Res., 24, 473 (1965).
Howard-Flanders, P., Levin, J., and Theriot, L., Radiat. Res., 18, 593 (1963).
Van Dyke, J. G., Biochem. Biophys. Res. Commun., 1, 54 (1959).
Johansen, I., and Howard-Flanders, P., Radiat. Research, 24, 184 (1965).
Alexander, P., Lett, J. T., and Dean, C. J., Prog. Biochem. Pharmacol., 1, (Karger, Basel/New York, 1965).
Hollaender, A., Stapleton, G. E., and Martin, G. L., Nature, 167, 103 (1951).
Howard-Flanders, P., Advances in Biological and Medical Physics, 6, 533 (Academic Press, New York, 1958).
Howard-Flanders, P., and Emmerson, P. T., Nature, 204, 1005 (1964).
Cramp, W. A., Nature, 206, 636 (1965).
Pittillo, R. F., Narkates, A. J., and Burns, J., Radiat. Res., 25, 401 (1965).
Cotton, I. M., and Lockingen, L. S., Proc. U.S. Nat. Acad. Sci., 50, 363 (1963).
Moroson, H., and Hernandez, B., Radiat. Res., 22 (1964).
Bridges, B. A., J. Gen. Microbiol., 26, 467 (1961).
Bruce, A. K., Abstract. Fb. 6, Radiation Research Society Fourteenth Annual Meeting, Coronado, California (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MOROSON, H., MARTIN, D. Radiosensitization of Escherichia coli by Methyl Hydrazine and the Effect of Oxygen. Nature 214, 304–306 (1967). https://doi.org/10.1038/214304a0
Issue Date:
DOI: https://doi.org/10.1038/214304a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.