Abstract
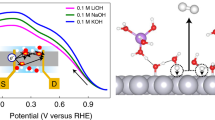
THE anodic oxidation of formic acid to carbon dioxide on platinum electrodes in acid electrolyte is strongly inhibited. This is caused by the firm adsorption of formic acid or one of its reaction products on the platinum surface1–4. At low potentials the oxidation rate (current density) decreases with time from a high initial value to a low equilibrium value. At this stage adsorbed hydrogen atoms can no longer be detected on the platinum surface. Only at potentials in excess of 300 mV does the oxidation proceed at an appreciable rate. At 70° C the polarization is approximately 100 mV smaller than at 30° C (Fig. 1).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brummer, S. B., and Makrides, A. C., J. Phys. Chem., 68, 1448 (1964).
Breiter, M. W., Electrochim. Acta, 10, 503 (1965).
Vielstich, W., and Vogel, U., Ber. Bunsengesell., 68, 688 (1964).
Giner, J., Electrochim. Acta, 9, 63 (1964).
Binder, H., Köhling, A., Krupp, H., Richter, K., and Sandstede, G., Chem. Eng. News, 42 (May 18, 1964); J. Electrochem. Soc., 112, 355 (1965).
Binder, H., Köhling, A., and Sandstede, G., Adv. Energ. Conv. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BINDER, H., KÖHLING, A. & SANDSTEDE, G. Acceleration by Adsorbed Sulphur and Selenium of the Electrochemical Oxidation of Formic Acid on Platinum Catalysts. Nature 214, 268–269 (1967). https://doi.org/10.1038/214268a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/214268a0
This article is cited by
-
Carbon monoxide adsorption on Pt/Pt electrodes modified with silver adatoms
Russian Journal of Electrochemistry (2005)
-
Enhanced Catalytic Activity of Platinum Electrodes resulting from Cathode Pretreatment
Nature (1968)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.