Abstract
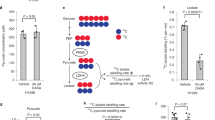
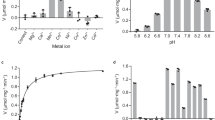
IN recent years several attempts have been made at a complete analysis of metabolic pathways. In comparing complex systems, be they intact cells or homogenates, with isolated enzymes, both similarities and differences1–3 have been found. So far no single pathway has been completely elucidated with respect to its kinetics. For this reason we turned to the erythrocyte, as representing both structurally and metabolically one of the simplest biological systems. One line of research which is to be reported here was concerned with the regulation of glycolysis on the level of lactate oxido-reductase. These studies were extended to ascites tumour cells. In view of the generally high activity of the lactate oxido-reductase (LDH) it may be assumed that the lactate/pyruvate system is at equilibrium within the cell1. Recently, however, deviations from the expected lactate/pyruvate ratios have been observed in brain homogenates3. We explored this question in a more detailed manner by studying the effect of pH on the formation of pyruvate and lactate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bücher, T., and Klingenberg, M., Angew. Chem., 70, 552 (1956).
Holzer, H., Schütz, G., and Lynen, F., Biochem. Z., 328, 251 (1956).
Lowry, O., Passoneau, J., Hasselberger, F., and Schulz, D., J. Biol. Chem., 239, 31 (1964).
Navazzio, F., Ernster, B., and Ernster, L., Biochim. Biophys. Acta, 26, 416 (1957).
Rapoport, S., and Ababei, L., Acta Biol. Med. Germ. (in the press).
Ababei, L., and Sarkar, S. R., Acta Biol. Med. Germ. (in the press).
Mehler, A. H., Kornberg, A., Grisolia, S., and Ochoa, S., J. Biol. Chem., 174, 961 (1948).
Meister, A., J. Biol. Chem., 184, 117 (1950).
Futterman, S., and Kinoshita, J. H., J. Biol. Chem., 234, 3174 (1959).
Wortman, B., and Schneider, A., Biochem. Biophys. Research Communs., 2, 179 (1960).
Rapoport, S., and Wagenknecht, C., Z. Physiol. Chem., 309, 296 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RAPOPORT, S., ABABEI, L., WAGENKNECHT, C. et al. Enzyme Regulatory Mechanisms at the Level of Lactate-oxidoreductase in Erythrocytes and Ascites Tumour Cells. Nature 208, 185–187 (1965). https://doi.org/10.1038/208185a0
Issue Date:
DOI: https://doi.org/10.1038/208185a0
This article is cited by
-
Glutamate dehydrogenase activity in normal and tumoural skin in humans
Experientia (1968)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.