Abstract
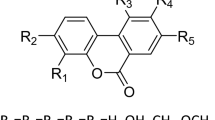
NITROPHENYL esters have proved to be very useful in examining hydrolytic enzymes1,2. The usually rapid rate of hydrolysis can be conveniently followed by the production of nitrophenolate ion which itself is measured by an increased absorption at 400 mµ. These esters appear to be hydrolysed at the same ‘active sites’ of the enzymes which are involved in the catalytic process with other synthetic or natural substrates3–6. Furthermore, when examining the effect of a given enzyme on a series of nitrophenyl esters with different substitutions in the acid portions, a pattern of specificity emerges which is fully comparable with that obtained with more conventional substrates5,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hartley, B. S., and Kilby, B. A., Biochem. J., 50, 672 (1952).
Bender, M. L., J. Amer. Chem. Soc., 84, 2582 (1962).
Spencer, T., and Sturtevant, J. M., J. Amer. Chem. Soc., 81, 1874 (1959).
Lorand, L., and Rule, N. G., Nature, 190, 722 (1961).
Lorand, L., Brannen, jun., W. T., and Rule, N. G., Arch. Biochem. Biophys., 96, 147 (1962).
Rule, N. G., and Lorand, L., Biochim. Biophys. Acta, 81, 130 (1964).
Zerner, B., and Bender, M. L., J. Amer. Chem. Soc., 85, 356 (1963).
Ploug, J., and Kjeldgaard, N. O., Biochim. Biophys. Acta, 24, 278 (1957).
White, W. F., and Mozen, M. M. (personal communication).
Remmert, L. F., and Cohen, P. P., J. Biol. Chem., 181, 431 (1949).
Troll, W., Sherry, S., and Wachman, J., J. Biol. Chem., 208, 85 (1954).
Martin, C. J., Golubow, J., and Axelrod, A. E., J. Biol. Chem., 234, 1718 (1959).
Kjeldgaard, N. O., and Ploug, J., Biochim. Biophys. Acta, 24, 283 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LORAND, L., MOZEN, M. Ester-hydrolysing Activity of Urokinase Preparations. Nature 201, 392–393 (1964). https://doi.org/10.1038/201392a0
Issue Date:
DOI: https://doi.org/10.1038/201392a0
This article is cited by
-
Crystalline Deposits in Striped Muscle in Xanthinuria
Nature (1969)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.