Abstract
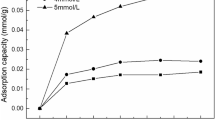
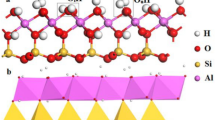
KEENAN et al.1 in an examination of adsorption of water vapour on several monoionic derivatives of kaolinite from Bath, South Carolina (Peerless No. 2, Vanderbilt Co., New York), found that—hysteresis being absent for all samples—lithium kaolinite showed the lowest water adsorption at any relative vapour pressure. Prior to the adsorption measurements (at 20° C), the samples were degassed under high vacuum at 70° C. Expressing the amount of water adsorbed per unit surface, these authors found identical values at any relative pressure for two samples of lithium kaolinite with different granulometric composition. They concluded that the adsorbed lithium ions have no effect on adsorption of water, and attributed this behaviour to the small ionic size of lithium, which permits the unhydrated ion to fit into the surface structure or to penetrate in the vacant octahedrally co-ordinated sites, thus becoming sterically hindered from interaction with water vapour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Keenan, A. G., Mooney, R. W., and Wood, L. A., J. Phys. Coll. Chem., 55, 1462 (1951).
Martin, R. T., Clays and Clay Minerals, 259 (Pergamon Press, New York, 1959).
Jurinak, J. J., J. Phys. Chem., 65, 62 (1961).
Jacobs, T. H. (to be published).
Jacobs, T. H., Nature, 182, 1086 (1958).
Grim, R. E., Clay Mineralogy, 129 (McGraw-Hill Book Co., Inc., New York, 1953).
Milliken, T. H., Mills, G. A., and Oblad, A. G., Disc. Farad. Soc., No. 8, 279 (1950).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
JACOBS, T. Surface Charge Density and Sorption of Water Vapour on Lithium Kaolinite. Nature 199, 481–482 (1963). https://doi.org/10.1038/199481a0
Issue Date:
DOI: https://doi.org/10.1038/199481a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.