Abstract
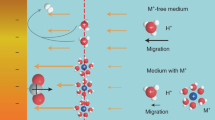
IN previous communications1,2 the influence of the nature and concentration of anions on the rate of electroreduction of Co(NH3)5Xn+ complexes has been reported. The influence exerted depends on the nature of the ligand X. This phenomenon has been explained by the assumption that the complex particle does not behave as a conducting sphere with uniform charge distribution (as assumed usually) but that the non-homogeneous charge distribution contributes considerably to the electrostatic free energy of activation and has to be taken into account. The ligand X represents the region of maximum non-homogeneity which induces an opposite non-homogeneity in the ionic atmosphere, the cations of the supporting electrolyte being preferentially attracted into the parts of the ionic atmosphere near the negative pole of the central particle. This increased concentration of cations shields the negative pole of the complex particle or might lead to the formation of an ion-pair between the positively charged complex particle and the cation. Both these effects make the attainment of the favourable orientation (that is, with ligand X removed from the positively charged electrode surface2) of the particle in the activated complex depolarizer-electrode2 much easier and increase the rate of electro-reduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Vlček, A. A., Disc. Farad. Soc., No. 29, 114 (1960).
Vlček, A. A., Advances in the Chemistry of the Coordination Compounds, edit. by Kirschner, S., 590 (Macmillan Co., New York, 1961).
Kuta, J., and Smoler, I., Z. Elekrochem., 64, 285 (1960).
Posey, F. A., and Taube, A., J. Amer. Chem. Soc., 79, 255 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VLČEK, A. Influence of Polyvalent Cations on the Rate of Electro-reductio n and Aquation of Co(NH3)5Xn+ Complexes. Nature 197, 786–787 (1963). https://doi.org/10.1038/197786a0
Issue Date:
DOI: https://doi.org/10.1038/197786a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.