Abstract
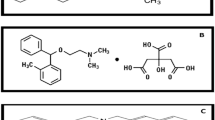
A NUMBER of analyses have been developed1, 2, 3 whereby citric acid can be estimated with varying degrees of sensitivity. Some1 are usable for only small quantities of citric acid in solution, while others2, 3 possess a considerably wider range but are complicated either by reagents or by the sensitivity of the determination at the higher levels. For the most part these methods are difficult to handle and vary in their sensitivity from day to day. The method of Cartier and Pin, for example, can be used to determine reasonably wide ranges (100–1200 µgm.) of citric acid; but the reagent used for the development of the colour reaction is light-sensitive and may interfere with the estimation. The methods of Natalson et al. and Buffa and Peters, on the other hand, are complicated by the fact that the reagent used to decolorize the permanganate (hydrogen peroxide) interferes with the colour reaction, and considerable care must be taken to remove all traces of it.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Natelson, N. E., Pincus, J. B., and Lugovog, J. K., J. Biol. Chem., 175, 745 (1948).
Cartier, D., and Pin, P., Bull. Soc. Chim. Biol., 31, 1176 (1949).
Buffa, P., and Peters, R. N., J. Physiol., 110, 488 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MACDONALD, R., WATERBURY, W. Colorimetric Estimation of Citric Acid. Nature 184, 988–989 (1959). https://doi.org/10.1038/184988a0
Issue Date:
DOI: https://doi.org/10.1038/184988a0
This article is cited by
-
Der Einfluss von Sorte und Standort auf die an der enzymatischen Verfärbung beteiligten Inhaltsstoffe der Kartoffel
Potato Research (1975)
-
Studies on a New Intramuscular Haematinic for Piglet Anaemia
Acta Veterinaria Scandinavica (1968)
-
Direkte densitometrische Methode zur Auswertung der Papierchromatogramme von Äpfelsäure, Weinsäure und Citronensäure in Obst
Zeitschrift für Lebensmittel-Untersuchung und -Forschung (1965)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.