Abstract
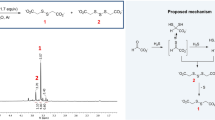
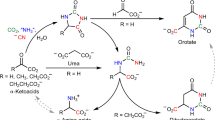
RECENT work in this laboratory demonstrated the occurrence of a new enzyme, aminomalonic acid decarboxylase, in the posterior silk-gland of silkworm and in rat liver1. The fact that this enzyme was first observed in silkworm, which has a remarkably high activity of glycine synthesis2, seems to suggest a physiological significance of the enzyme in the biosynthesis of glycine, although natural occurrence of this amino-acid has hitherto not been reported.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shimura, K., Nagayama, H., and Kikuchi, A., Nature, 177, 935 (1956).
Koide, F., Shishido, T., Nagayama, H., and Shimura, K., J. Agric. Chem. Soc. Japan, 30, 283 (1956).
Braunstein, A. E., Enzymologia, 7, 25 (1939).
Green, D. E., Leloir, L. F., and Nocito, V., J. Biol. Chem., 161, 559 (1945).
Krueger, R., Helv. Chim. Acta, 32, 238 (1949).
Cavallini, D., and Frontali, N., Biochim. Biophys. Acta, 13, 439 (1954).
Braunstein, A. E., Adv. Protein Chem., 3, 1 (1947).
Campbell, jun., L. L., J. Bacteriol., 71, 81 (1956).
Meister, A., J. Biol. Chem., 206, 587 (1954).
Stafford, H. A., Plant Physiol., 31, 135 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
NAGAYAMA, H., MURAMATSU, M. & SHIMURA, K. Enzymatic Formation of Aminomalonic Acid from Ketomalonic Acid. Nature 181, 417–418 (1958). https://doi.org/10.1038/181417a0
Issue Date:
DOI: https://doi.org/10.1038/181417a0
This article is cited by
-
Towards creating an extended metabolic model (EMM) for E. coli using enzyme promiscuity prediction and metabolomics data
Microbial Cell Factories (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.