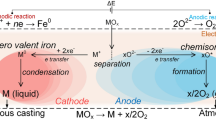
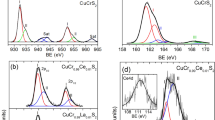
Abstract
IT is well established that high-purity chromium can be produced by electrolysing an aqueous solution of chromic acid containing a small sulphate addition, and that a significant factor governing the purity of such material is the temperature of the electrolyte1. Oxygen and nitrogen contents of 0.02 and 0.001 weight per cent respectively or better can be attained provided the bath temperature is high, that is, in the vicinity of 80° C. Under optimum conditions the metallic impurity is extremely low.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brenner, A., Burkhead, P., and Jennings, C., J. Res. Nat. Bur. Stand., 40, 31 (1948). Greenaway, H. T., J. Inst. Metals, 83, 121 (1954–55).
Henderson, F., Quaass, S. T., and Wain, H. L., J. Inst. Metals, 83, 126 (1954–55).
Wain, H. L., Henderson, F., Johnstone, S. T. M., and Louat, N., J. Inst. Metals (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RYAN, N., HENDERSON, F., JOHNSTONE, S. et al. Some Properties of Chromium deposited from an Electrolyte containing Fluoride. Nature 180, 1406–1407 (1957). https://doi.org/10.1038/1801406b0
Issue Date:
DOI: https://doi.org/10.1038/1801406b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.