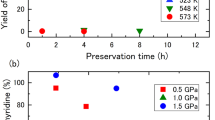
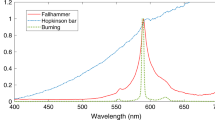
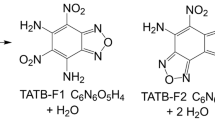
Abstract
HYDROGEN azide is an extremely unstable and endothermic compound, and even in the absence of oxygen it may detonate violently. Nitrogen, hydrogen and ammonia are its decomposition products, and stoichiometrically decomposition must lie between the extremes of equations (1) and (2). Experimentally, it is found that the slow thermal decomposition of gaseous hydrogen azide is heterogeneous1 and follows equation (2), yielding mainly ammonia, while the rapid, explosive decomposition initiated by sparking2 follows equation (1), yielding no ammonia: 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Meyer, R., and Schumacher, H. J., Z. phys. Chem., A, 170, 33 (1934).
Gunther, P., Meyer, R., and Müller-Skjøld, F., Z. phys. Chem., A, 175, 154 (1935).
Campbell, H. C., and Rice, O. K., J. Amer. Chem. Soc., 57, 1044 (1935). Rice, O. K., J. Chem. Phys., 7, 700 (1939).
Leermakers, J. A., J. Amer. Chem. Soc., 55, 2719 (1933).
Gray, P., Proc. Roy. Soc., A, 221, 462 (1954).
Gray, P., and Waddington, T. C., Proc. Roy. Soc., A, 235, 106, 481 (1956).
Pannetier, G., et al., J. Chim. Phys., 48, 222 (1951). Bull. Soc. Chim. France, 1068 (1954); C.R. Acad. Sci., Paris, 240, 958 (1955);
Rice, F. C., and Freamo, M., J. Amer. Chem. Soc., 73, 5529 (1951); 75, 548 (1953). Mador, I. L., and Williams, M. C., J. Chim. Phys., 21, 1627 (1954). Whittle, E., Dows, D. A., and Pimentel, G. C., J. Chem. Phys., 21, 1943 (1954); 22, 1606 (1955).
Gray, P., and Waddington, T. C., Research, 8, S56, (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GRAY, P., WADDINGTON, T. Spontaneous Ignition of Gaseous Hydrogen Azide. Nature 179, 576–577 (1957). https://doi.org/10.1038/179576a0
Issue Date:
DOI: https://doi.org/10.1038/179576a0
This article is cited by
-
Ignition of gaseous mixtures of hydrazoic acid and various diluents
Combustion, Explosion, and Shock Waves (1970)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.