Abstract
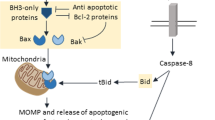
Mitochondria play a key part in the regulation of apoptosis (cell death)1,2. Their intermembrane space contains several proteins that are liberated through the outer membrane in order to participate in the degradation phase of apoptosis3,4,5,6,7,8,9. Here we report the identification and cloning of an apoptosis-inducing factor, AIF5, which is sufficient to induce apoptosis of isolated nuclei. AIF is a flavoprotein of relative molecular mass 57,000 which shares homology with the bacterial oxidoreductases; it is normally confined to mitochondria but translocates to the nucleus when apoptosis is induced. Recombinant AIF causes chromatin condensation in isolated nuclei and large-scale fragmentation of DNA. It induces purified mitochondria to release the apoptogenic proteins cytochrome c and caspase-9. Microinjection of AIF into the cytoplasm of intact cells induces condensation of chromatin, dissipation of the mitochondrial transmembrane potential, and exposure of phosphatidylserine in the plasma membrane. None of these effects is prevented by the wide-ranging caspase inhibitor known as Z-VAD.fmk. Overexpression of Bcl-2, which controls the opening of mitochondrial permeability transition pores, prevents the release of AIF from the mitochondrion but does not affect its apoptogenic activity. These results indicate that AIF is a mitochondrial effector of apoptotic cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kroemer, G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med. 3, 614–620 (1997).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Zamzami, N. et al. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183, 1533–1544 (1996).
Liu, X. S., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 (1996).
Susin, S. A. et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 184, 1331–1342 (1996).
Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 (1997).
Vander Heiden, M. G., Chandal, N. S., Williamson, E. K., Schumacker, P. T. & Thompson, C. B. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91, 627–637 (1997).
Mancini, M. et al. The caspase-3 precursor has a cytosolic and mitochondrial distribution: Implications for apoptotic signaling. J. Cell Biol. 140, 1485–1495 (1998).
Susin, S. A. et al. Mitochondrial release of caspases-2 and -9 during the apoptotic process. J. Exp. Med. 189, 381–394 (1999).
Ducret, A., Van Ostveen, I., Eng, J. K., Yates, J. R. & Aebersold, R. High-throughput protein characterization by automated reverse-phase chromatography electrospray tandem mass spectrometry. Protein Sci. 7, 706–719 (1998).
Claros, M. G. & Vincens, P. Computation method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241, 779–786 (1996).
Cedano, J., Aloy, P., Pérez-Pons, J. A. & Quero, E. Relation between amino acid composition and cellular location of proteins. J. Mol. Biol. 266, 594–600 ((1997)).
Boulikas, T. Nuclear localization signals (NLS). Crit. Rev. Euk. Gene Exp. 3, 193–227 (1993).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apopotic protease cascade. Cell 91, 479–489 (1997).
Yasuhara, N. et al. Essential role of active nuclear transport in apoptosis. Genes to Cells 2, 55–64 (1997).
Enari, M. et al. Acaspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391, 43–50 (1998).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signalling pathways. EMBO J. 17, 1675–1687 (1998).
Kluck, R. M. et al. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16, 4639–4649 (1997).
Robinson, K. M. & Lemire, B. D. Arequirement for matrix processing peptidase but not for mitochondrial chaperonin in the covalent attachment of FAD to yeast succinate dehydrogenase flavoprotein. J. Biol. Chem. 271, 4061–4067 (1996).
Liu, X., Zou, H., Slaughter, C. & Wang, X. DFF, a heterodimeric protein that functions downstream of caspase 3 to trigger DNA fragmentation during apoptosis. Cell 89, 175–184 (1997).
Samejima, K. et al. Transition from caspase-dependent to caspase-independent mechanisms at the onset of apoptotic execution. J. Cell Biol. 143, 225–239 (1998).
Oberhammer, F. et al. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12, 3679–3684 (1993).
Lagarkova, M. A., Iarvaia, O. V. & Razin, S. V. Large-scale fragmentation of mammalian DNA in the course of apoptosis proceeds via excision of chromosomal DNA loops and their oligomers. J. Biol. Chem. 270, 20239–20241 (1995).
Trbovich, A. M. et al. High and low molecular weight DNA cleavage in ovarian granulosa cells: characterization and protease modulation in intact cells and in cell-free nuclear autodigestion assays. Cell Death Differ. 5, 38–49 (1998).
Susin, S. A. et al. The central executioner of apoptosis. Multiple links between protease activation and mitochondria in Fas/Apo-1/CD95- and ceramide-induced apoptosis. J. Exp. Med. 186, 25–37 (1997).
Bossy-Wetzel, E., Newmeyer, D. D. & Green, D. R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17, 37–49 (1998).
Marzo, I. et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281, 2027–2031 (1998).
Enari, M., Hase, A. & Nagata, S. Apoptosis by a cytosolic extract from Fas-activated cells. EMBO J. 14, 5201–5208 (1995).
Wada, J. & Kanwar, Y. S. Characterization of mammalian translocase of inner mitochondrial membrane (Tim44) isolated from diabetic newborn mouse kidney. Proc. Natl Acad. Sci. USA 95, 144–149 (1998).
Zhu, W. et al. Bcl-2 mutants with restricted subcellular localization reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 15, 4130–4141 (1996).
Acknowledgements
We thank P. M. Alzari for suggestions, M. Geuskens for electron microscopy, G.Salvesen for caspase-8, D. Andrews for Rat-1 cells, and S. Arya and S. Chung for expression constructs. This work was supported by grants from ANRS, ARC, CNRS, FF, FRM, INSERM, LNC, and PRFMMIP (to G.K.), the NIH, the NSF Science and Technology Center for Molecular Biotechnology (to R.A.), and Amgen (to D.P.S. and J.M.P.). S.A.S. and I.M. hold postdoctoral fellowships from the European Commission and from the Spanish Ministry of Science, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Susin, S., Lorenzo, H., Zamzami, N. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 (1999). https://doi.org/10.1038/17135
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/17135
This article is cited by
-
Quantitative Proteomics Reveals Manganese Alleviates Heat Stress of Broiler Myocardial Cells via Regulating Nucleic Acid Metabolism
Biological Trace Element Research (2024)
-
Superwettable interface towards biodetection in confined space
Nano Research (2024)
-
p20BAP31 induces cell apoptosis via both AIF caspase-independent and the ROS/JNK mitochondrial pathway in colorectal cancer
Cellular & Molecular Biology Letters (2023)
-
Cytoplasmic Endonuclease G promotes nonalcoholic fatty liver disease via mTORC2-AKT-ACLY and endoplasmic reticulum stress
Nature Communications (2023)
-
Environmental hypoxia induces apoptosis in large yellow croaker Larimichthys crocea via both intrinsic and extrinsic pathways
Journal of Oceanology and Limnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.