Abstract
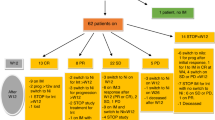
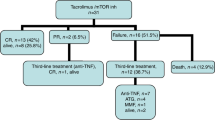
GVHD remains a significant complication of allogeneic hematopoietic stem cell transplantation. Tumor necrosis factor-α (TNF-α) is a major mediator of GVHD pathogenesis. Infliximab inhibits the binding of TNF-α with its cellular receptors and has been associated with encouraging responses in adults with severe GVHD. We retrospectively evaluated the efficacy and safety of infliximab 10 mg/kg i.v. once a week for a median of eight doses (range 1–162) in 24 children with steroid-resistant GVHD. The overall response rate in 22 evaluable children was 82% (12 CR+6 PR). Among those patients with acute GVHD, both skin and gastrointestinal involvement responded well to infliximab; however long-term outcome was poor. While infliximab may be useful to acutely control GVHD manifestations, GVHD recurs commonly upon discontinuation of infliximab. Within 100 days of the final infliximab dose, 77% of patients had bacterial infections, 32% had viral infections and 13.6% had probable or proven non-candidal invasive fungal infections. Infliximab appears to be well-tolerated and to have activity in steroid-resistant GVHD. Controlled studies to assess the pharmacokinetics and most effective dosing regimen of infliximab for the treatment of GVHD are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant 2000; 6: 441–447.
Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scime R, Locatelli F et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian group for bone marrow transplantation. Blood 1998; 92: 2288–2293.
Gratma JW, Jansen J, Lopovich RA, Tanke HJ, Goldstein G, Zwaan FE . Treatment of acute graft versus host disease with monoclonal antibody OKT3. Transplantation 1984; 38: 469–474.
Martin PJ, Hansen JA, Anacetti C, Zutter M, Durnam D, Storb R . Treatment of acute graft-versus-host disease with anti CD3 monoclonal antibody. Am J Kidney Disease 1988; 11: 149–152.
Ferrara JL, Levy R, Chao NJ . Pathophysiologic mechanisms of acute graft-versus-host disease. Biol Blood Marrow Transplant 1999; 5: 347–356.
Piguet PF, Grau GE, Allet B, Vassalli P . Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs-host disease. J Exp Med 1987; 166: 1280–1289.
Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL . Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997; 90: 3204–3213.
Holler E, Kolb HJ, Hintermeirer-Knabe R, Mittermuller J, Thierfelder S, Kaul M et al. Role of tumor necrosis factor alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant Proc 1993; 25: 1234–1236.
Herve P, Flesch M, Tiberghien P, Wijdenes J, Racadot E, Bordigoni P et al. Phase I-II trial of monoclonal anti-tumor necrosis factor alpha antibody for the treatment of refractory severe acute graft-versus-host disease. Blood 1992; 79: 3362–3368.
Knight DM, Trinh H, Junming L, Siegel S, Shealy D, McDonough M et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol 1993; 30: 1443–1453.
Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol 1999; 163: 1521–1528.
Couriel D, Hicks K, Ipolitti C, de Lima M, Donato M, Martin T et al. Infliximab for the treatment of graft-versus-host disease in allogeneic transplant recipients: an update. Blood 2000; 96: 400a (abstr. 1678).
Redei I, Knoche J, Tanner AR, Langston AA, Lonial S, Cherry JK et al. Salvage therapy with infliximab for patients with severe acute and chronic GVHD. Blood 2001; 98: 399a (abstr. 1724).
Kobbe G, Schneider P, Rohr U, Fenk R, Neumann F, Aivado M et al. Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse antiTNF alpha antibody. Bone Marrow Transplant 2001; 28: 47–49.
Magalhaes-Silverman M, Lee C, Hohl R, Becker A, Gingrich R . Treatment of severe steroid refractory acute graft versus host disease with infliximab. Blood 2001; 98: 359b (abstr. 5208).
Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB . Infliximab for steroid-refractory acute GVHD: a case series. Am J Hematol 2003; 74: 119–124.
Yamane T, Yamamura R, Aoyama Y, Nakamae H, Hasegawa T, Sakamoto C et al. Infliximab for the treatment of severe steroid refractory acute graft-versus-host disease in three patients after allogeneic hematopoietic transplantation. Leuk Lymphoma 2003; 44: 2095–2097.
Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C et al. Tumor necrosis factor-α blockade for the treatment of acute GVHD. Blood 2004; 104: 649–654.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
Nevo S, Enger C, Swan V, Wojno KJ, Fuller AK, Altomonte V et al. Acute bleeding after allogeneic bone marrow transplantation: association with graft versus host disease and effect on survival. Transplantation 1999; 67: 681–689.
Gassas A, Sung L, Doyle JJ, Clarke JT, Saunders EF . Life-threatening pulmonary hemorrhages post bone marrow transplantation in Hurler syndrome. Report of three cases and review of the literature. Bone Marrow Transplant 2003; 32: 213–215.
REMICADE (Infliximab). Package Insert, March 2006 version.
Sleight B, Shenoy S, Haight A, Vora R, Prabhakar U, Schultz K et al. Pharmacokinetics of anti tumor Necrosis factor antibody (Infliximab) for pediatric acute graft versus host disease involving the gastrointestinal tract. Biol Blood Marrow Transplant 2006; 12: p69 (abstr. 197).
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476.
Menachem Y, Avidan B, Lavy A, Lang A, Bardan E, Fidder H et al. Increasing the infliximab dose is beneficial in Crohn's disease patients who responded to a lower dose and relapsed. Digestion 2005; 72: 124–128.
Wewer V, Riis L, Vind I, Husby S, Munkholm P, Paerregaard A . Infliximab dependency in a national cohort of children with Crohn's disease. J Pediatr Gastroenterol Nutr 2006; 42: 40–45.
Rutgeerts P, K'Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH et al. Efficacy and safety of retreatment with anti-tumor necrosis factor (infliximab) to maintain remission in Crohn's Disease. Gastroenterology 1999; 117: 761–769. 29.
Fukuda T, Boeckh M, Carter R, Sandmaier BM, Maris MB, Maloney DG et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 2003; 102: 827–833.
Baden LR . Prevention and therapy of fungal infections in bone marrow transplantation. Leukemia 2003; 17: 1038–1041.
Baddley J, Stroud T, Salzman D . Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis 2001; 32: 1319–1324.
Wald A, Leisenring W, van Burik JA, Bowden RA . Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997; 175: 1459–1466.
Jantunen E, Ruutu P, Niskanen L, Volin L, Parkkali T, Koukila-Kahkola P et al. Incidence and risk factors for invasive fungal infections in allogeneic BMT recipients. Bone Marrow Transplant 1997; 19: 801–808.
Marr KA, Carter RA, Crippa F, Wald A, Corey L . Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34: 909–917.
Marty FM, Lee SJ, Fahey MM, Alyea EP, Soiffer RJ, Antin JH et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood 2003; 102: 2768–2776.
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. NEJM 2001; 345: 1098–1104.
Cook KR, Yanik G . Acute lung injury after allogeneic stem cell transplantation: is the lung a target of acute graft-versus-host disease? Bone Marrow Transplant 2004; 34: 753–765.
Author information
Authors and Affiliations
Corresponding author
Additional information
These data were presented at the American Society for Blood and Marrow Transplantation meeting at Keystone, CO, USA, in January 2004.
Rights and permissions
About this article
Cite this article
Sleight, B., Chan, K., Braun, T. et al. Infliximab for GVHD therapy in children. Bone Marrow Transplant 40, 473–480 (2007). https://doi.org/10.1038/sj.bmt.1705761
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705761
Keywords
This article is cited by
-
Evaluation of infliximab as second-line treatment of acute graft versus host disease -validating response on day 7 and 28 as predictors of survival
Bone Marrow Transplantation (2018)
-
State-of-the-art acute and chronic GVHD treatment
International Journal of Hematology (2015)
-
Successful response to infliximab of recurrent pericardial graft versus host disease in a pediatric patient
Bone Marrow Transplantation (2013)
-
Implications of TNF-α in the pathogenesis and management of GVHD
International Journal of Hematology (2011)
-
Sudden loss of the GVL effect following use of the TNF inhibitor infliximab in a chronic myelogenous leukemia patient with chronic GVHD
Bone Marrow Transplantation (2010)