Abstract
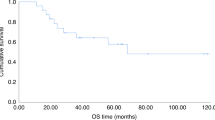
High-risk primary breast cancer patients treated with high-dose chemotherapy (HDC) and stem cell support (SCS) have shown prolonged disease-free survival (DFS) in many studies; however, only one trial has demonstrated an overall survival benefit (OS). We hypothesize that the period following myeloablative therapy is ideal for immunologic manipulation and studied the effects of two different methods of immunotherapy following HDC with SCS aimed at the window of immune reconstitution. Seventy-two women with high-risk stage II or III breast cancer were randomized following HDC to receive either interleukin 2 (IL-2) at 1 million units/m2 SQ daily for 28 days or combined cyclosporine A (CsA) at 1.25 mg/kg intravenously daily from day 0 to +28 and interferon gamma (IFN-γ) 0.025 mg/m2 SQ every 2 days from day +7 to +28. At a median follow-up of 67 months, no significant difference was observed in DFS or OS between the two treatment groups. The IL-2 arm had a 59% DFS (95% CI (0.45, 0.78)) and a 72% OS (95% CI (0.58, 0.88)) at 5 years. The CsA/INF-γ arm had a similar outcome with a 55% DFS (95% CI (0.40, 0.76)) and a 78% OS (95% CI (0.65, 0.94)) at 5 years. Treatment was well tolerated, without increased toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Roche H, Viens P, Biron P, Lotz JP, Asselain B . High-dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control 2003; 10: 42–47.
Crown J, Lind M, Gould A, Verrill M, Twelves C, Coleman R et al. High-dose chemotherapy with autograft(PBP) support is not superior to cyclophosphamide, methotrexate and 5-FU following doxorubicin induction in patients with breast cancer and 4 or more involved axillary lymph nodes: the Anglo-Celtic I study. Proc Amer Soc Clin Oncol 2002; 21: 42a (abstract 166).
Nitz UA, Mohrmann S, Fischer J, Lindemann W, Berdel WE, Jackisch C et al. Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet 2005; 366: 1935–1944.
Rodenhuis S, Bontenbal M, Beex LV, Wagstaff J, Richel DJ, Nooij MA et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med 2003; 349: 7–16.
Tallman MS, Gray R, Robert NJ, LeMaistre CF, Osborne CK, Vaughan WP et al. Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med 2003; 349: 17–26.
Peters WP, Rosner GL, Vredenburgh JJ, Shpall EJ, Crump M, Richardson PG et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: a report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol 2005; 23: 2191–2200.
Hanrahan EO, Broglio K, Frye D, Buzdar AU, Theriault RL, Valero V et al. Randomized trial of high-dose chemotherapy and autologous hematopoietic stem cell support for high-risk primary breast carcinoma: follow-up at 12 years. Cancer 2006; 106: 2327–2336.
Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979; 300: 1068–1073.
Glazier A, Tutschka PJ, Farmer ER, Santos GW . Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med 1983; 158: 1–8.
Hess AD, Fischer AC, Beschorner WE . Effector mechanisms in cyclosporine A-induced syngeneic graft-versus-host disease. Role of CD4+ and CD8+ T lymphocyte subsets. J Immunol 1990; 145: 526–533.
Geller RB, Esa AH, Beschorner WE, Frondoza CG, Santos GW, Hess AD . Successful in vitro graft-versus-tumor effect against an Ia-bearing tumor using cyclosporine-induced syngeneic graft-versus-host disease in the rat. Blood 1989; 74: 1165–1171.
Sorokin R, Kimura H, Schroder K, Wilson DH, Wilson DB . Cyclosporine-induced autoimmunity. Conditions for expressing disease, requirement for intact thymus, and potency estimates of autoimmune lymphocytes in drug-treated rats. J Exp Med 1986; 164: 1615–1625.
Hess A, Noga S . Cyclosporine-induced syngeneic graft-versus-host-disease: an immunotherapeutic approach after autologous bone marrow transplantation. Int J Cell Cloning 1992; 10: 179.
Hess AD, Thoburn CJ . Immunobiology and immunotherapeutic implications of syngeneic/autologous graft-versus-host disease. Immunol Rev 1997; 157: 111–123.
Miura Y, Ueda M, Zeng W, Wang H, Takami A, Yamazaki H et al. Induction of autologous graft-versus-host disease with cyclosporin A after peripheral blood stem cell transplantation: analysis of factors affecting induction. J Allergy Clin Immunol 2000; 106: S51–S57.
Jones RJ, Vogelsang GB, Hess AD, Farmer ER, Mann RB, Geller RB et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet 1989; 1: 754–757.
Vogelsang G, Bitton R, Piantadosi S, Altomonte V, Horn T, Jones R et al. Immune modulation in autologous bone marrow transplantation: cyclosporine and gamma-interferon trial. Bone Marrow Transplant 1999; 24: 637–640.
Kennedy MJ, Vogelsang GB, Beveridge RA, Farmer ER, Altomonte V, Huelskamp AM et al. Phase I trial of intravenous cyclosporine to induce graft-versus-host disease in women undergoing autologous bone marrow transplantation for breast cancer. J Clin Oncol 1993; 11: 478–484.
Kennedy MJ, Vogelsang GB, Jones RJ, Farmer ER, Hess AD, Altomonte V et al. Phase I trial of interferon gamma to potentiate cyclosporine-induced graft-versus-host disease in women undergoing autologous bone marrow transplantation for breast cancer. J Clin Oncol 1994; 12: 249–257.
Cheever MA, Greenberg PD, Fefer A, Gillis S . Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med 1982; 155: 968–980.
Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL . Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med 1985; 161: 1169–1188.
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316: 889–897.
Ault KA, Antin JH, Ginsburg D, Orkin SH, Rappeport JM, Keohan ML et al. Phenotype of recovering lymphoid cell populations after marrow transplantation. J Exp Med 1985; 161: 1483–1502.
Armitage RJ, Goldstone AH, Richards JD, Cawley JC . Lymphocyte function after autologous bone marrow transplantation (BMT): a comparison with patients treated with allogeneic BMT and with chemotherapy only. Br J Haematol 1986; 63: 637–647.
Singer CR, Tansey PJ, Burnett AK . T lymphocyte reconstitution following autologous bone marrow transplantation. Clin Exp Immunol 1983; 51: 455–460.
Welte K, Ciobanu N, Moore MA, Gulati S, O'Reilly RJ, Mertelsmann R . Defective interleukin 2 production in patients after bone marrow transplantation and in vitro restoration of defective T lymphocyte proliferation by highly purified interleukin 2. Blood 1984; 64: 380–385.
Meropol NJ, Porter M, Vaickus RPPL, Loewen GM, Creaven PJ, Wilkes KA et al. Low dose interleukin-2 (IL-2): daily subcutaneous injection results in selective in vivo expansion of natural killer (NK) cells with minimal toxicity. Proc Am Clin Oncol 1994; 13: 296.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 2002; 62: 4854–4859.
Soiffer RJ, Murray C, Cochran K, Cameron C, Wang E, Schow PW et al. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood 1992; 79: 517–526.
Candido K, Shimizu K, McLaughlin J, Kunkel P, Fuller R, Redman B et al. Local administration of dendritic cells inhibits established breast tumor growth: implication for apoptosis-inducing agents. Cancer Res 2001; 61: 228–236.
Tong Y, Song W, Crystal RG . Combined intratumoral injection of bone marrow-derived dendritic cells systemic chemotherapy to treat pre-existing murine tumors. Cancer Res 2001; 61: 7530–7535.
Avigan D, Vasir D, Gong J, Wu Z, Borges V, Uhl L et al. Vaccination with dendritic cell (DC)-breast cancer fusions induces tumor specific immunity and clinical response in patients with metastatic breast cancer. Proc Am Soc Hem 2002; 100, abstract 2651.
Nesselhut T, Matthes C, Marx D, Chang R, Nesselhut J, Cillien N et al. Cancer therapy with immature monocyte-derived dendritic cells in patients with advanced breast cancer. Proc Am Soc Clin Oncol 2005; 23, abstract 2528.
Chui S, Clay T, Hobeika A, Venturi C, Osada T, Khan S et al. Dendritic cell vaccination following high dose chemotherapy with autologous stem cell support for breast cancer: long term follow-up. Proc Amer Soc Clin Oncol 2003; 22, abstract 682.
Schuetz F, Diel I, Beckhove P, Ehlert K, Schirrmacher V, Schneeweiss A et al. Cellular immunotherapy in late stage breast cancer patients with reactivated autologous Memory T-cells derived from bone marrow. Proc Am Soc Clin Oncol 2005; 23: 714.
Lum LG, Sen M . Activated T-cell and bispecific antibody immunotherapy for high-risk breast cancer. Bench to bedside. Acta Haematol 2001; 105: 130–136.
Carella AM, Beltrami G, Corsetti MT, Nati S, Musto P, Scalzulli P et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet 2005; 366: 318–320.
Bishop MR, Fowler DH, Marchigiani D, Castro K, Kasten-Sportes C, Steinberg SM et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol 2004; 22: 3886–3892.
Folkman J . Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg 1972; 175: 409–416.
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31.
Tosetti F, Ferrari N, De Flora S, Albini A . Angioprevention: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J 2002; 16: 2–14.
Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res 2000; 6: 1–10.
Acknowledgements
We thank our patients and their families for supporting this trial. We also thank the nursing staff of 5GS for their professionalism and compassion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vahdat, L., Cohen, D., Zipin, D. et al. Randomized trial of low-dose interleukin-2 vs cyclosporine A and interferon-γ after high-dose chemotherapy with peripheral blood progenitor support in women with high-risk primary breast cancer. Bone Marrow Transplant 40, 267–272 (2007). https://doi.org/10.1038/sj.bmt.1705692
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705692