Summary:
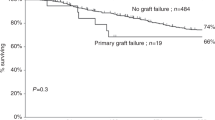
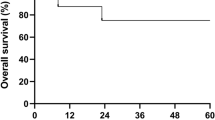
The occurrence of post-transplant lymphoproliferative disorder (PTLD) in relation to immunosuppressive treatment was determined in 257 patients treated with non-T-cell-depleted allogeneic stem cell transplantation from an HLA-matched sibling (173 patients) or unrelated donor (84 patients). The conditioning consisted of total body irradiation and cyclophosphamide (myeloablative conditioning, 250 patients), or fludarabine combined with cyclophosphamide or a single 2 Gy dose of TBI (nonmyeloablative conditioning, seven patients). In transplantations from an unrelated donor, the patients also received antithymocyte globulin (ATG). The prophylaxis against graft-versus-host disease (GVHD) consisted of cyclosporine A, methotrexate, and methylprednisolone. The autopsy reports of deceased patients were systematically reviewed, and the autopsy materials of cases suggestive of PTLD were re-examined histologically for Epstein–Barr virus (EBV). Nineteen patients with EBV-positive PTLD were identified, of whom six had been transplanted from a sibling donor and 13 from an unrelated donor. All the patients who developed PTLD had been given ATG either for the treatment of steroid-resistant acute GVHD (all PTLD patients with a sibling donor and one with an unrelated donor), or as part of the conditioning (all patients with an unrelated donor). In conclusion, in transplantations from an HLA-identical donor with a non-T-cell-depleted graft, the risk of PTLD correlated strongly with the intensity of the immunosuppressive treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cohen JI . Epstein-Barr virus infection. N Engl J Med 2000; 343: 481–492.
Swinnen LJ . Diagnosis and treatment of transplant-related lymphoma. Ann Oncol 2000; 11 (Suppl. 1): 45–48.
Paya CV, Fung JJ, Nalesnik MA et al. Epstein–Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International Consensus Development Meeting. Transplantation 1999; 68: 1517–1525.
Lucas KG, Burton RL, Zimmerman SE et al. Semiquantitative Epstein–Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood 1998; 91: 3654–3661.
Curtis RE, Travis LB, Rowlings PA et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999; 94: 2208–2216.
van Esser JW, van der Holt B, Meijer E et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood 2001; 98: 972–978.
Zutter MM, Martin PJ, Sale GE et al. Epstein–Barr virus lymphoproliferation after bone marrow transplantation. Blood 1988; 72: 520–529.
Micallef IN, Chhanabhai M, Gascoyne RD et al. Lymphoproliferative disorders following allogeneic bone marrow transplantation: the Vancouver experience. Bone Marrow Transplant 1998; 22: 981–987.
Stevens SJ, Verschuuren EA, Pronk I et al. Frequent monitoring of Epstein–Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood 2001; 97: 1165–1171.
Benkerrou M, Jais JP, Leblond V et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood 1998; 92: 3137–3147.
Papadopoulos EB, Ladanyi M, Emanuel D et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994; 330: 1185–1191.
Ifthikharuddin JJ, Mieles LA, Rosenblatt JD et al. CD-20 expression in post-transplant lymphoproliferative disorders: treatment with rituximab. Am J Hematol 2000; 65: 171–173.
Kuehnle I, Huls MH, Liu Z et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 2000; 95: 1502–1505.
Chetty R, Biddolph S, Kaklamanis L et al. Bcl-2 protein is strongly expressed in post-transplant lymphoproliferative disorders. J Pathol 1996; 180: 254–258.
Faye A, Quartier P, Reguerre Y et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. Br J Haematol 2001; 115: 112–118.
Van Esser JW, Niesters HG, Van Der Holt B et al. Prevention of Epstein–Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002; 99: 4364–4369.
Gustafsson A, Levitsky V, Zou JZ et al. Epstein–Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood 2000; 95: 807–814.
Rooney CM, Smith CA, Ng CY et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998; 92: 1549–1555.
Ruutu T, Volin L, Parkkali T et al. Cyclosporine, methotrexate, and methylprednisolone compared with cyclosporine and methotrexate for the prevention of graft-versus-host disease in bone marrow transplantation from HLA-identical sibling donor: a prospective randomized study. Blood 2000; 96: 2391–2398.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Thomas ED, Storb R, Clift RA et al. Bone-marrow transplantation (second of two parts). N Engl J Med 1975; 292: 895–902.
Aalto S, Juvonen E, Tarkkanen J et al. Lymphoproliferative disease after allogeneic stem cell transplantation – pre-emptive diagnosis by quantification of Epstein–Barr virus DNA in serum. J Clin Virol (in press).
Gaber AO, First MR, Tesi RJ et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation 1998; 66: 29–37.
Volin L, Juvonen E, Parkkali T et al. Anti-thymocyte globulin (ATG) in the conditioning for unrelated donor transplantation: comparison of two regimens. Hematol J 2002; 3 (Suppl. 1): 24.
Acknowledgements
This study was financially supported by the Finnish Cancer Research Foundation, the HUCH (EVO) Fund, and the Finnish Technology Advancement Fund.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juvonen, E., Aalto, S., Tarkkanen, J. et al. High incidence of PTLD after non-T-cell-depleted allogeneic haematopoietic stem cell transplantation as a consequence of intensive immunosuppressive treatment. Bone Marrow Transplant 32, 97–102 (2003). https://doi.org/10.1038/sj.bmt.1704089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704089
Keywords
This article is cited by
-
Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes
Bone Marrow Transplantation (2020)
-
Post Transplant Lymphoproliferative Disorder
Indian Journal of Hematology and Blood Transfusion (2020)
-
Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation
Leukemia (2011)
-
High incidence of post transplant lymphoproliferative disorder after antithymocyte globulin-based conditioning and ineffective prediction by day 28 EBV-specific T lymphocyte counts
Bone Marrow Transplantation (2011)
-
Epstein–Barr virus-related lymphoproliferative disorder induced by equine anti-thymocyte globulin therapy
Medical Oncology (2011)