Abstract
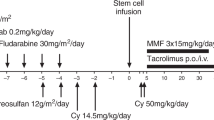
We investigated whether a T cell-reduced allogeneic stem cell transplant (SCT) with minimal conditioning and subsequent donor lymphocyte infusions (DLI) could reduce the incidence and severity of GVHD while retaining stable engraftment. Five patients with hematological malignancies (three MM, one CLL, one Chediak-Higashi syndrome) were conditioned with TBI (200 cGy). One patient additionally received fludarabine (120 mg/m2). CsA and mofetyl-mycophenolate (MMF) were administered to prevent GVHD. All patients were grafted with >3 × 106/kg highly purified CD34+ cells together with 2 × 106/kg CD3+ cells (three patients) or 1 × 105/kg CD3+ cells (two patients). Quick hematopoietic recovery and initial mixed donor chimerism was observed. Treatment-related toxicity was minimal in all but one patient who died of treatment-refractory GVHD on day 112. The four other patients only achieved partial donor T cell chimerism. BM and PBMC donor chimerism was lost between day 40 and 209 despite DLI. Three patients are alive with disease and one is in CR. We conclude that T cell-reduced SCT using 200 cGy as the conditioning regimen does not result in stable hematopoietic engraftment. Predominant donor T cell chimerism is not a prerequisite for initial allogeneic hematopoietic proliferation. However for sustained long-term engraftment it is of major importance. Bone Marrow Transplantation (2001) 28, 157–161.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Carella AM, Champlin R, Slavin S et al. Mini-allografts: ongoing trials in humans (editorial) Bone Marrow Transplant 2000 25: 345–350
Carella AM, Giralt S, Slavin S . Low intensity regimens with allogeneic hematopoietic stem cell transplantation as treatment of hematologic neoplasia Haematologica 2000 85: 304–313
Storb R, Yu C, Zaucha JM et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant Blood 1999 94: 2523–2529
Storb R, Yu C, Barnett T et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation Blood 1999 94: 1131–1136
Storb R, Yu C, Wagner JL et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation Blood 1997 89: 3048–3054
Yu C, Seidel K, Nash RA et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts Blood 1998 91: 2581–2587
Kottaridis PD, Milligan DW, Chopra R et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation Blood 2000 96: 2419–2425
Horowitz MM, Gale RP, Sondel PM et al. Graft-versus-leukemia reactions after bone marrow transplantation Blood 1990 75: 555–562
Truitt RL, Atasoylu AA . Contribution of CD4+ and CD8+ T cells to graft-versus-host disease and graft-versus-leukemia reactivity after transplantation of MHC-compatible bone marrow Bone Marrow Transplant 1991 8: 51–58
Van Deerlin VM, Leonard DG . Bone marrow engraftment analysis after allogeneic bone marrow transplantation Clin Lab Med 2000 20: 197–225
Stolz W, Graubner U, Gerstmeier J et al. Chediak–Higashi syndrome: approaches in diagnosis and treatment Curr Probl Dermatol 1989 18: 93–100
Knauf W, Fietz T, Schrezenmeier H, Thiel E . CD34 selected alloPBSCT and adoptive immunotherapy Bone Marrow Transplant 2000 25: (Suppl. 2) S2–S5
Novitzky N, Rubinstein R, Hallett JM et al. Bone marrow transplantation depleted of T cells followed by repletion with incremental doses of donor lymphocytes for relapsing patients with chronic myeloid leukemia: a therapeutic strategy Transplantation 2000 69: 1358–1363
Craddock C, Bardy P, Kreiter S et al. Short report: Engraftment of T-cell-depleted allogeneic haematopoietic stem cells using a reduced intensity conditioning regimen Br J Haematol 2000 111: 797–800
Georges GE, Storb R, Thompson JD et al. Adoptive immunotherapy in canine mixed chimeras after nonmyeloablative hematopoietic cell transplantation Blood 2000 95: 3262–3269
Childs R, Clave E, Contentin N et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses Blood 1999 94: 3234–3241
Acknowledgements
We would like to thank Beate Stradmann-Bellinghausen and Jutta Lummer for their excellent technical assistance. This work was supported by a grant from the Deutsche Krebshilfe (No. 70–2428-Schm 2). Martin Schuler is a fellow of the Dr Mildred Scheel Stiftung für Krebsforschung.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kreiter, S., Winkelmann, N., Schneider, P. et al. Failure of sustained engraftment after non-myeloablative conditioning with low-dose TBI and T cell-reduced allogeneic peripheral stem cell transplantation. Bone Marrow Transplant 28, 157–161 (2001). https://doi.org/10.1038/sj.bmt.1703107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703107
Keywords
This article is cited by
-
Response and toxicity of donor lymphocyte infusions following T-cell depleted non-myeloablative allogeneic hematopoietic SCT from 3–6/6 HLA matched donors
Bone Marrow Transplantation (2009)
-
Novel approaches in allogeneic stem cell transplantation
Current Oncology Reports (2006)
-
Reduced-intensity allogeneic hematopoietic stem cell transplantation for acute leukemias: ‘what is the best recipe?’
Bone Marrow Transplantation (2005)
-
Reduced-intensity conditioning followed by allografting of CD34-selected stem cells and ?106/kg T cells may have an adverse effect on transplant-related mortality
Annals of Hematology (2005)
-
Lineage-specific chimaerism after stem cell transplantation in children following reduced intensity conditioning: potential predictive value of NK cell chimaerism for late graft rejection
Leukemia (2003)