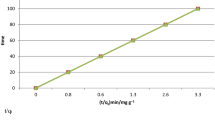
Abstract
AMONG chromatographic adsorbents there is continually in progress a search for substances of higher adsorbing capacity and/or increased selectivity of adsorption. We have found that a number of sparingly soluble inorganic solids exhibit a small but definite binding affinity for amino-acids, and have attempted to relate such adsorption to certain physical properties of the adsorbent. The electrokinetic properties of both natural and artificial samples of several such solids have been investigated1, and from these results it could be expected that such differences would be reflected in comparative affinities for binding amino-acids. Such has been found to be the case for the two amino-acids discussed here, namely, aspartic acid and glycine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Buchanan, A. S., and Heymann, E., Nature, 161, 649 (1948); Proc. Roy. Soc., A., 195, 150 (1948). O'Connor, D. J., and Buchanan, A. S. (unpublished data).
Hamoir, G. C. M., Biochem. J., 39, 485 (1945).
Bryant, P., and O'Connor, E. J., Aust. J. Sci., 13, 111 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'CONNOR, D., BRYANT, F. Adsorption of Amino-acids on Sparingly Soluble Inorganic Solids. Nature 170, 84–85 (1952). https://doi.org/10.1038/170084a0
Issue Date:
DOI: https://doi.org/10.1038/170084a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.