Abstract
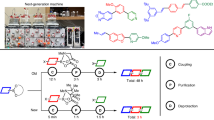
FLAVOGLAUCIN, C19H28O3, m.p. 109–110°, and auroglaucin, C19H22O3, m.p. 152–153°, are two pigments, yellow and red respectively, isolated by Raistrick and his collaborators1 from Aspergillus glaucus, and later found by A. Quilico and L. Panizzi2 in the mycelium of Aspergillus echinulatus, where they are accompanied by a neutral colourless substance, C28H39O2N3 (echinulin)3. On the basis of detailed chemical investigations by Robinson, Raistrick, Todd, Cruickshank and others4–6, structures like () and () were ascribed to the pigments, the position and the structure of the chain (or chains) present in the residuo C5H10 (which contains a double bond) still being undetermined. The existence of the group (n)C7H15CO was inferred from the fact that n-octoic acid was obtained from () on oxidation with hydrogen peroxide and sodium hydroxide (by Dakin's reaction); the easy formation of a Schiff's base and phenylhydrazone derivatives both from () and () points to the presence of a free ortho position ciose to the carbonyl chain. The quinol structure was demonstrated by the absorption spectrum in the ultra-violet of tetrahydroflavoglaucin () dimethyl ether. As dihydroflavoglaucin, C19H30O3, proved to be identical with octohydroauroglaucin, the two pigments can differ only by the existence of three double bonds in the C8 side-chain, as formulæ () and () indicate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gould, B. G., and Raistrick, H., Biochem. J., 28, 1640 (1934).
Quilico, A., and Panizzi, L., Ber., 76, 348 (1943).
Quilico, A., Pauizzi, L., and Rosnati, V., Gazz. Chim. Ital., 78, 111 (1948).
Raistrick, H., Robinson, R., and Todd, A. R., J. Chem. Soc., 80 (1937).
Cruickshank, J. H., Raistrick, H., and Robinson, R., J. Chem. Soc., 2056 (1938).
Cruickshank, J. H., and Robinson, R., J. Chem. Soc., 2064 (1938).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
QUILICO, A., PANIZZI, L. & MUGNAINI, E. Structure of Flavoglaucin and Auroglaucin. Nature 164, 26–27 (1949). https://doi.org/10.1038/164026a0
Issue Date:
DOI: https://doi.org/10.1038/164026a0
This article is cited by
-
Eurotium (Aspergillus) repens metabolites and their biological activity
Folia Microbiologica (1978)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.