Abstract
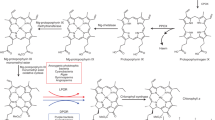
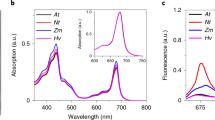
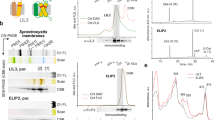
When etiolated angiosperm seedlings break through the soil after germination, they are immediately exposed to sunlight, but at this stage they are unable to perform photosynthesis1. In the absence of chlorophyll a and chlorophyll b, two other porphyrin species cooperate as the basic light-harvesting structure of etiolated plants. Protochlorophyllide a and protochlorophyllide b (ref. 2) form supramolecular complexes with NADPH and two closely related NADPH:protochlorophyllide oxidoreductase (POR) proteins—PORA and PORB (ref. 3)—in the prolamellar body of etioplasts. Here we report that these light-harvesting POR–protochlorophyllide complexes, named LHPP, are essential for the establishment of the photosynthetic apparatus and also confer photoprotection on the plant. They collect sunlight for rapid chlorophyll a biosynthesis and, simultaneously, dissipate excess light energy in the bulk of non-photoreducible protochlorophyllide b. Based on this dual function, it seems that LHPP provides the link between skotomorphogenesis and photosynthesis that is required for efficient de-etiolation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Granick, S. The structural and functional relationship between heme and chlorophyll. Harvey Lect. 44, 220–245 (1950).
Shedbalkar, V. P., Ioannides, I. M. & Rebeiz, C. A. Chloroplast biogenesis. Detection of monovinyl protochlorophyllide b in plants. J. Biol. Chem. 266, 17151–17157 (1991).
Holtorf, H., Reinbothe, S., Reinbothe, C., Bereza, B. & Apel, K. Two routes of chlorophyllide synthesis that are differentially regulated by light in barley. Proc. Natl Acad. Sci. USA 92, 3254–3258 (1995).
Griffiths, W. T. Reconstitution of chlorophyll formation by isolated etioplast membranes. Biochem. J. 174, 681–692 (1978).
Reinbothe, S. & Reinbothe, C. The regulation of enzymes involved in chlorophyll biosynthesis. Eur. J. Biochem. 237, 323–343 (1995).
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994).
Palsson, L. O., Spangfort, M. D., Gulbinas, V. & Gillbro, T. Ultrafast chlorophyll b to chlorophyll a excitation energy transfer in the isolated light harvesting complex, LHCII, of green plants: implications for the organisation of chlorophylls. FEBS Lett. 339, 134–138 (1994).
Ide, J. P., Klug, D. R., Kühlbrandt, W., Georgi, L. & Porter, G. The state of detergent-solubilized light-harvesting chlorophyll-a/b protein complex as monitored by picosecond time-resolved fluorescence and circular dichroism. Biochim. Biophys. Acta 893, 349–364 (1987).
Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. PCR Protocols (Academic, San Diego, (1990).
Krieg, P. A. & Melton, D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned DNAs. Nucleic Acids Res. 12, 7057–7070 (1984).
Apel, K., Santel, H.-J., Redlinger, T. E. & Falk, H. The protochlorophyllide holochrome of barley. Isolation and characterization of the NADPH:protochlorophyllide oxidoreductase. Eur. J. Biochem. 111, 251–258 (1980).
Reinbothe, S., Runge, S., Reinbothe, C., van Cleve, B. & Apel, K. Substrate-dependent transport of the NADPH:protochlorophyllide oxidoreductase into isolated plastids. Plant Cell 7, 161–172 (1995).
Schoch, S., Helfrich, M., Wiktorsson, B., Sundqvist, C., Rüdiger, W. & Ryberg, M. Photoreduction of protopheophorbide with NADPH-protochlorophyllide oxidoreductase from etiolated wheat (Triticum aestivum). Eur. J. Biochem. 229, 291–298 (1995).
Lebedev, N., van Cleve, B., Armstrong, G. A. & Apel, K. Chlorophyll synthesis in a de-etiolated (det340) mutant of Arabidopsis without NADPH-protochlorophyllide (Pchlide) oxidoreductase (POR) A and photoactive Pchlide-F655. Plant Cell 7, 2081–2090 (1995).
Ryberg, M. & Sundqvist, C. in Chlorophylls (ed. Scheer, H.) 587–612 (CRC Press, Boca Raton, (1991).
Ryberg, M., Sandelius, A. S. & Selstam, E. Lipid composition of prolamellar bodies and prothylakoids of wheat etioplasts. Physiol. Planta 57, 555–560 (1983).
Armstrong, G. A., Runge, S., Frick, G., Sperling, U. & Apel, K. Identification of protochlorophyllide oxidoreductases A and B: a branched pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliania. Plant Physiol. 108, 1505–1517 (1995).
von Wettstein, D., Gough, S. & Kannangara, C. G. Chlorophyll biosynthesis. Plant Cell 7, 1039–1057 (1995).
Quail, P. H., Boylan, M. T., Parks, B. M., Short, T. W., Xu, Y. & Wagner, D. Phytochromes: photosensory perception and signal transduction. Science 268, 675–680 (1995).
Kendrick, R. E. & Kronenberg, H. M. (eds) Photomorphogenesis in Plants (Kluwer, Dordrecht, (1991).
Schulz, R. et al. Nucleotide sequence of a cDNA coding for the NADPH-protochlorophyllide oxidoreductase (POR) of barley (Hordeum vulgare L.) and expression in Escherichia coli. Mol. Gen. Genet. 217, 355–361 (1989).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Reinbothe, C., Apel, K. & Reinbothe, S. Alight-induced protease from barley plastids degrades NADPH:protochlorophyllide oxidoreductase complexed with chlorophyllide. Mol. Cell. Biol. 15, 6206–6212 (1995).
Towbin, M., Staehelin, T. & Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA 76, 4350–4354 (1979).
Acknowledgements
We thank S. Schoch for a gift of ZnPPa and ZnPPb. This work is dedicated to R.Mache on the occasion of his 65th birthday.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinbothe, C., Lebedev, N. & Reinbothe, S. A protochlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 397, 80–84 (1999). https://doi.org/10.1038/16283
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16283
This article is cited by
-
Privet golden leaves adapt unexpectedly well to light changes
Horticulture, Environment, and Biotechnology (2020)
-
NADPH:protochlorophyllide oxidoreductase B (PORB) action in Arabidopsis thaliana revisited through transgenic expression of engineered barley PORB mutant proteins
Plant Molecular Biology (2017)
-
Cascade exciton-pumping engines with manipulated speed and efficiency in light-harvesting porous π-network films
Scientific Reports (2015)
-
The myth of interconnected plastids and related phenomena
Protoplasma (2015)
-
Cytokinin and abscisic acid control plastid gene transcription during barley seedling de-etiolation
Plant Growth Regulation (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.