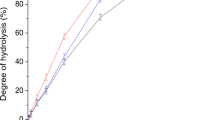
Abstract
BOTH pure potato phosphorylase1 and crystalline muscle phosphorylase2 produce from glucose-1-phosphate a non-branched polysaccharide containing only α-1,4-glucosidic links and resembling amylose. By the action of extracts of yeast3, of heart, brain or liver4, branched polysaccharides are obtained containing up to 10 per cent of α-1,6-glucosidic linkages and resembling glycogen. In 1942, Meyer and Bernfeld5 reported the presence of an enzyme in yeast which brings about the phosphorolysis of the terminal α-1,6-glucosidic links of residual dextrin (the final degradation product of amylopectin by β-amylase). A purified potato phosphorylase was not able to effect this reaction. It was therefore concluded5 that there are two different phosphorylases, one of which effects the fission or synthesis of α-1,4-glucosidic links, the other the α-1,6-glucosidic links involved in branching. Cori6 has found an enzyme in liver extract and also in heart extract Which he calls the ‘branching factor', and which is able to produce glycogen by simultaneous action with the crystalline muscle phosphorylase. Haworth, Peat and Bourne7 have described a thermolabile factor which they term the 'Q' and to which they attribute the property of synthesizing the α-1,6-glucosidic links in amylopectin. Their 'Q enzyme' has, in addition, an amylatic action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hanes, C. S., Proc. Roy. Soc., B, 128, 421 (1940); 129, 174 (1940). Hassid, W. Z., and McCready, R. M., Amer. Chem. Soc., 63, 2171 (1941).
Hassid, W. Z., Cori, G. T., and McCready, R. M., J. Biol. Chem., 148, 89 (1943).
Schäffner, A., and Specht, H., Naturwiss., 26, 494 (1938). Kiessling, W., Naturwiss., 27, 129 (1939).
Cori, G. T., and Cori, C. F., J. Biol. Chem., 135, 733 (1940). Bear, R. S., and Cori, C. F., J. Biol. Chem., 140, 111 (1941).
Meyer, K. H., and Bernfeld, P., Helv. Chim. Acta, 25, 399 (1942); 25, 404 (1942). See also Meyer, K. H., in Advances of Enzymology, 3, 129 (1943).
Cori, G. T., and Cori, C. F., J. Biol. Chem., 151, 57 (1943).
Haworth, W. N., Peat, S., Bourne, E. J., Macey, A., and Barker, S. A., Nature, 154, 236 (1944); J. Chem. Soc., 877, 882 (1945); Nature, 161, 127 (1948).
Bernfeld, P., and Gürtler, P., Helv. Chim. Acta, 31, 106 (1948).
Katz, J., Hassid, W. Z., and Doudoroff, M., Nature, 161, 96 (1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BERNFELD , P., MEUTÉ MÉ DIAN, A. Isophosphorylase. Nature 162, 297–298 (1948). https://doi.org/10.1038/162297b0
Issue Date:
DOI: https://doi.org/10.1038/162297b0
This article is cited by
-
Microbiological production of enzymes and their industrial applications
Economic Botany (1951)
-
Amylopectin and Glycogen : Products of Irreversible and Reversible Synthesis
Nature (1949)
-
Isophosphorylase and the Formation of Branched Polysaccharides
Nature (1948)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.