Abstract
Objective:
To investigate the effects of regular chilli ingestion on some indicators of metabolic and vascular function.
Design:
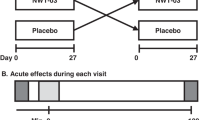
A randomized cross-over dietary intervention study.
Setting:
Launceston, Australia.
Subjects:
Healthy free-living individuals.
Intervention:
Thirty-six participants (22 women and 14 men), aged 46±12 (mean±s.d.) years; BMI 26.4±4.8 kg/m2, consumed 30 g/day of a chilli blend (55% cayenne chilli) with their normal diet (chilli diet), and a bland diet (chilli-free) for 4 weeks each. Metabolic and vascular parameters, including plasma glucose, serum lipids and lipoproteins, insulin, basal metabolic rate, blood pressure, heart rate, augmentation index (AIx; an indicator of arterial stiffness), and subendocardial-viability ratio (SEVR; a measure of myocardial perfusion), were measured at the end of each diet. In a sub-study, during week 3 of each dietary period, the vascular responses of 15 subjects to glyceryl-trinitrate (GTN) and salbutamol were also studied.
Results:
For the whole group, there were no significant differences between any of the measured parameters when compared at the end of the two dietary periods. When analysed separately, men had a lower resting heart rate (P=0.02) and higher SEVR (P=0.05) at the end of the chilli diet than the bland diet. In the sub-study, baseline AIx on the chilli diet was lower (P<0.001) than on the bland diet, but there was no difference in the effects of GTN and salbutamol between the two diets.
Conclusion:
Four weeks of regular chilli consumption has no obvious beneficial or harmful effects on metabolic parameters but may reduce resting heart rate and increase effective myocardial perfusion pressure time in men.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Belza A, Frandsen E, Kondrup J (2006). Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: a placebo-controlled, double-blind 8-week intervention in obese subjects. Int J Obes (London).
Belza A, Jessen AB (2005). Bioactive food stimulants of sympathetic activity: effect on 24-h energy expenditure and fat oxidation. Eur J Clin Nutr 59, 733–741.
Buckberg GD, Fixler DE, Archie JP, Hoffman JI (1972). Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30, 67–81.
Chen IJ, Yeh JL, Lo YC, Sheu SH, Lin YT (1996). Capsinolol: the first beta-adrenoceptor blocker with an associated calcitonin gene-related peptide releasing activity in the heart. Br J Pharmacol 119, 7–14.
Danchin N, Aly S (2004). Heart rate reduction: a potential target for the treatment of myocardial ischaemia. Therapie 59, 511–515.
Eckel RH, Grundy SM, Zimmet PZ (2005). The metabolic syndrome. Lancet 365, 1415–1428.
Ferrell WR, Wong BB, Lockhart JC, Ramsay JE (2004). Gender differences in regional cutaneous microcirculatory responses to capsaicin. Fundam Clin Pharmacol 18, 195–200.
Fokkema DS, VanTeeffelen JW, Dekker S, Vergroesen I, Reitsma JB, Spaan JA (2005). Diastolic time fraction as a determinant of subendocardial perfusion. Am J Physiol Heart Circ Physiol 288, H2450–H2456.
Fragasso G, Palloshi A, Piatti PM, Monti L, Rossetti E, Setola E et al. (2004). Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J Cardiovasc Pharmacol 44, 340–347.
Friedewald WT, Levy RI, Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502.
Gillum RF, Makuc DM, Feldman JJ (1991). Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J 121, 172–177.
Henry CJ, Emery B (1986). Effect of spiced food on metabolic rate. Hum Nutr Clin Nutr 40, 165–168.
Kannel WB, Kannel C, Paffenbarger Jr RS, Cupples LA (1987). Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 113, 1489–1494.
Kawada T, Hagihara K, Iwai K (1986a). Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr 116, 1272–1278.
Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K (1986b). Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med 183, 250–256.
Lee CY, Kim M, Yoon SW, Lee CH (2003). Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother Res 17, 454–458.
Lejeune MP, Kovacs EM, Westerterp-Plantenga MS (2003). Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr 90, 651–659.
Lim K, Yoshioka M, Kikuzato S, Kiyonaga A, Tanaka H, Shindo M et al. (1997). Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Med Sci Sports Exerc 29, 355–361.
Matsumoto T, Miyawaki C, Ue H, Kanda T, Yoshitake Y, Moritani T (2001). Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes Res 9, 78–85.
Matsumoto T, Miyawaki C, Ue H, Yuasa T, Miyatsuji A, Moritani T (2000). Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J Nutr Sci Vitaminol (Tokyo) 46, 309–315.
Moller DE, Kaufman KD (2005). Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 56, 45–62.
Munce TA, Kenney WL (2003). Age-specific skin blood flow responses to acute capsaicin. J Gerontol A Biol Sci Med Sci 58, 304–310.
Negulesco JA, Lohse CL, Hrabovsky EE, Boggs MT, Davis DH (1989). Dihydrocapsaicin (DC) protects against serum hyperlipidemia in guinea pigs fed a cholesterol-enriched diet. Artery 16, 174–188.
Negulesco JA, Noel SA, Newman HA, Naber EC, Bhat HB, Witiak DT (1987). Effects of pure capsaicinoids (capsaicin and dihydrocapsaicin) on plasma lipid and lipoprotein concentrations of turkey poults. Atherosclerosis 64, 85–90.
Oroszi G, Szilvassy Z, Nemeth J, Tosaki A, Szolcsanyi J (1999). Interplay between nitric oxide and CGRP by capsaicin in isolated guinea-pig heart. Pharmacological Research 40, 125–128.
O’Rourke MF, Gallagher DE (1996). Pulse wave analysis. J Hypertens Suppl 14, S147–S157.
Roberts RG, Westerman RA, Widdop RE, Kotzmann RR, Payne R (1992). Effects of capsaicin on cutaneous vasodilator responses in humans. Agents Act 37, 53–59.
Saito A, Nakamura K, Hori Y, Yamamoto M (1999). Effects of capsaicin on serum triglycerides and free fatty acid in olive oil treated rats. Int J Vitam Nutr Res 69, 337–340.
Saito A, Nakamura K, Hori Y, Yamamoto M (2000). Effects of capsaicin on biliary free fatty acids in rats. Int J Vitam Nutr Res 70, 19–23.
Schaefer EJ, Lichtenstein AH, Lamon-Fava S, Contois JH, Li Z, Rasmussen H et al. (1995a). Efficacy of a National Cholesterol Education Program Step 2 Diet in Normolipidemic and Hypercholesterolemic Middle-Aged and Elderly Men and Women. Arterioscler Thromb Vasc Biol 15, 1079–1085.
Schaefer EJ, Lichtenstein AH, Lamon-Fava S, McNamara JR, Schaefer MM, Rasmussen H et al. (1995b). Body weight and low-density lipoprotein cholesterol changes after consumption of a low-fat ad libitum diet. JAMA 274, 1450–1455.
Segal KR, Gutin B, Albu J, Pi-Sunyer FX (1987). Thermic effects of food and exercise in lean and obese men of similar lean body mass. Am J Physiol 252, E110–E117.
Srinivasan MR, Chandrasekhara N (1992). Comparative influence of vanillin & capsaicin on liver & blood lipids in the rat. Indian J Med Res 96, 133–135.
Sundell J (2005). Obesity and diabetes as risk factors for coronary artery disease: from the epidemiological aspect to the initial vascular mechanisms. Diabetes Obes Metab 7, 9–20.
Suzuki T, Wada S, Tomizawa N, Kamata R, Saito S, Sato I et al. (1998). A possible role of nitric oxide formation in the vasodilatation of rabbit ear artery induced by a topically applied Capsaicin analogue. J Vet Med Sci 60, 691–697.
Tani Y, Fujioka T, Sumioka M, Furuichi Y, Hamada H, Watanabe T (2004). Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J Nutr Sci Vitaminol (Tokyo) 50, 351–355.
Watanabe T, Kawada T, Iwai K (1988). Effect of capsaicin pretreatment on capsaicin-induced catecholamine secretion from the adrenal medulla in rats. Proc Soc Exp Biol Med 187, 370–374.
Watanabe T, Kawada T, Yamamoto M, Iwai K (1987). Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem Biophys Res Commun 142, 259–264.
Weir JB (1949). New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109, 1–9.
Westerbacka J, Tamminen M, Cockcroft J, Yki-Jarvinen H (2004). Comparison of in vivo effects of nitroglycerin and insulin on the aortic pressure waveform. Eur J Clin Invest 34, 1–8.
Wilkinson IB, Cockcroft JR, Webb DJ (1998). Pulse wave analysis and arterial stiffness. J Cardiovasc Pharmacol 32, S33–S37.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ (2000). The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525, 263–270.
Yoshioka M, Doucet E, Drapeau V, Dionne I, Tremblay A (2001). Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br J Nutr 85, 203–211.
Yoshioka M, Imanaga M, Ueyama H, Yamane M, Kubo Y, Boivin A et al. (2004). Maximum tolerable dose of red pepper decreases fat intake independently of spicy sensation in the mouth. Br J Nutr 91, 991–995.
Yoshioka M, Lim K, Kikuzato S, Kiyonaga A, Tanaka H, Shindo M et al. (1995). Effects of red-pepper diet on the energy metabolism in men. J Nutr Sci Vitaminol (Tokyo) 41, 647–656.
Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M et al. (1999). Effects of red pepper on appetite and energy intake. Br J Nutr 82, 115–123.
Yoshioka M, St-Pierre S, Suzuki M, Tremblay A (1998). Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br J Nutr 80, 503–510.
Acknowledgements
We are grateful to MasterFoods, Australia for providing the ‘Freshly Chopped Chilli’. We thank Dr Rob Fassett and his team at the Launceston General Hospital, for looking after our study participants during the drug tests. We also thank Ms Jane Pittaway, Dr Andrew Williams and Dr Tom Hartley for their advice, time and assistance with biochemical analysis and setting up the metabolic cart.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: KDK Ahuja.
Contributors: MB and DG designed the study, discussed the data and corrected the manuscript. IR provided statistical support. KA recruited the subjects, conducted the study, performed all the analysis, and wrote the manuscript.
Rights and permissions
About this article
Cite this article
Ahuja, K., Robertson, I., Geraghty, D. et al. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur J Clin Nutr 61, 326–333 (2007). https://doi.org/10.1038/sj.ejcn.1602517
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602517
Keywords
This article is cited by
-
Association between frequency of spicy food consumption and hypertension: a cross-sectional study in Zhejiang Province, China
Nutrition & Metabolism (2021)
-
Effects of Capsicum annuum supplementation on the components of metabolic syndrome: a systematic review and meta-analysis
Scientific Reports (2020)
-
Sex-dependent difference in the association between frequency of spicy food consumption and risk of hypertension in Chinese adults
European Journal of Nutrition (2019)
-
Capsaicinoids: a spicy solution to the management of obesity?
International Journal of Obesity (2016)
-
Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans
European Journal of Nutrition (2013)