Abstract
Objective:
Due to inconsistent results based on dietary intake data, unsaturated fatty acids in red blood cell (RBC) membranes and diet were used to investigate their association with allergic sensitisation and allergic rhinitis.
Design:
Cross-sectional, population-based study.
Setting:
Bavarian Nutrition Survey II (2002–03), Germany.
Subjects:
A total of 568 adult participants, 325 women and 243 men.
Methods:
By means of logistic regression models, the relation of fatty acids to (i) allergic sensitisation as defined by means of specific serum immunoglobulin E analysis (CAPSX1 class ≥2), and (ii) self-reported allergic rhinitis was examined.
Results:
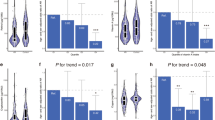
A high cell membrane level of eicosapentaenoic acid (EPA, 20:5 n-3) was inversely associated with allergic sensitisation, the adjusted odds ratio (OR) and 95% confidence interval (95% CI) were 0.52 (0.30–0.90) for the highest (vs lowest) quartile. A similar effect was observed for allergic rhinitis with an OR (95% CI) of 0.50 (0.24–1.03; P=0.027 for trend). A higher dietary intake of alpha-linolenic acid (ALA, 18:3 n-3) was associated with a decreased risk of allergic sensitisation and allergic rhinitis with ORs (95% CIs) of 0.51 (0.28–0.93) and 0.43 (0.20–0.93), respectively, in the highest quartiles. No other dietary or cell membrane unsaturated fatty acid was significantly associated with the outcome variables, nor was the n-6/n-3 ratio. The strongest effects were observed among subjects under the age of 40 y.
Conclusions:
In this cross-sectional study among adults, a high content of n-3 fatty acids in RBC membranes (EPA) or in the diet (ALA) is associated with a decreased risk of allergic sensitisation and allergic rhinitis.
Sponsorship:
The study was supported by funds of the Kurt-Eberhard-Bode-Stiftung and the Bavarian Ministry of Environment, Health and Consumer Protection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arab L (2003): Biomarkers of fat and fatty acid intake. J. Nutr. 133 (Suppl 3), S925–S932.
Arab L & Akbar J (2002): Biomarkers and the measurement of fatty acids. Public Health Nutr. 5, 865–871.
Black PN & Sharpe S (1997): Dietary fat and asthma: is there a connection? Eur. Respir. J. 10, 6–12.
Bolte G, Frye C, Hoelscher B, Meyer I, Wjst M & Heinrich J (2001): Margarine consumption and allergy in children. Am. J. Respir. Crit. Care. Med. 163, 277–279.
Bousquet J, Van Cauwenberge P & Khaltaev N (2001): Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immunol. 108 (Suppl 5), S147–S334.
Burr ML, Butland BK, King S & Vaughan-Williams E (1989): Changes in asthma prevalence: two surveys 15 years apart. Arch. Dis. Child. 64, 1452–1456.
Butte W (1983): Rapid method for the determination of fatty acid profiles from fats and oils using trimethylsulphonium hydroxide for transesterification. J. Chrom. A 261, 142–145.
Calder PC (2001): Polyunsaturated fatty acids, inflammation, and immunity. Lipids 36, 1007–1024.
Calder PC (2003): Polyunsaturated fatty acids and cytokine profiles: a clue to the changing prevalence of atopy? Clin. Exp. Allergy 33, 412–415.
Droste JH, Kerhof M, de Monchy JG, Schouten JP & Rijcken B (1996): Association of skin test reactivity, specific IgE, total IgE, and eosinophils with nasal symptoms in a community-based population study. The Dutch ECRHS Group. J. Allergy Clin. Immunol. 97, 922–932.
Duchen K, Yu G & Bjorksten B (1998): Atopic sensitization during the first year of life in relation to long chain polyunsaturated fatty acid levels in human milk. Pediatr. Res. 44, 478–484.
Dunder T, Kuikka L, Turtinen J, Rasanen L & Uhari M (2001): Diet, serum fatty acids, and atopic diseases in childhood. Allergy 56, 425–428.
Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG & Precsott SL (2003): Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J. Allergy Clin. Immunol. 112, 1178–1184.
Filipiak B, Heinrich J, Nowak D & Wichmann HE (2001): The distribution in specific IgE and the prevalence of allergic symptoms in 25–64-years old inhabitants of an eastern and a western German city—results from Augsburg and Erfurt. Eur. J. Epidemiol. 17, 77–84.
Folch J, Lees M & Sloane Stanley GH (1957): A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Gibney MJ & Hunter B (1993): The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur. J. Clin. Nutr. 47, 255–259.
Golik A, Weissgarten J, Evans S, Cohen N, Averbukh Z, Zaidenstein R, Cotariu D & Modai D (1996): Erythrocyte Na+, K+ and Ca2+, Mg(2+)-ATPase activities in hypertensives on angiotensin-converting enzyme inhibitors. Clin. Biochem. 29, 249–254.
Heinrich J, Holscher B, Bolte G & Winkler G (2001): Allergic sensitization and diet: ecological analysis in selected European cities. Eur. Respir. J. 17, 395–402.
Heinrich J, Hoelscher B, Frye C, Meyer I, Wjst M & Wichmann HE (2002): Trends in prevalence of atopic diseases and allergic sensitization in children in Eastern Germany. Eur. Respir. J. 19, 1040–1046.
Jump DB (2002): Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr. Opin. Lipidol. 13, 155–164.
Katan M, Deslypere JP, van Birgelen APJ, Penders M & Zegwaard M (1997): Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J. Lipid. Res. 38, 2012–2022.
Kompauer I, Demmelmair H, Koletzko B, Bolte G, Linseisen J & Heinrich J (2004): n6/n3 hypothesis and allergies: biologically plausible, but not confirmed. Eur. J. Med. Res. 9, 378–382.
Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A & Jorgensen T (2000): Increasing prevalence of specific IgE to aeroallergens in an adult population: two cross-sectional surveys 8 years apart: the Copenhagen Allergy Study. J. Allergy Clin. Immunol. 106, 247–252.
Linseisen J, Schulze MB, Saadatian-Elahi M, Kroke A, Miller AB & Boeing H (2003): Quantity and quality of dietary fat, carbohydrate, and fiber intake in the german EPIC cohorts. Ann. Nutr. Metab. 47, 37–46.
Mihrshahi S, Peat JK, Marks GB, Mellis CM, Tovey ER, Webb K, Britton WJ and Leeder SR & Childhood Asthma Prevention Study (2003): Eighteen-month outcomes of house dust mite avoidance and dietary fatty acid modification in the Childhood Asthma Prevention Study (CAPS). J. Allergy Clin. Immunol. 111, 162–168.
Nafstad P, Nystad W, Magnus P & Jaakkola JJ (2003): Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J. Asthma 40, 343–348.
Nagakura T, Matsuda S, Shichijyo K, Sugimoto H & Hata K (2000): Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur. Respir. J. 16, 861–865.
Nagel G, Nieters A, Becker N & Linseisen J (2003): The influence of the dietary intake of fatty acids and antioxidants on hay fever in adults. Allergy 58, 1277–1284.
Sanders TA (2000): Polyunsaturated fatty acids in the food chain in Europe. Am. J. Clin. Nutr. 71 (Suppl 1), S176–S178.
Schwartz J & Weiss ST (1990): Dietary factors and their relation to respiratory symptoms—The Second National Health and Nutrition Examination Survey. Am. J. Epidemiol. 132, 67–76.
Sibbald B & Rink E (1991): Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax 46, 895–901.
Slimani N, Deharveng G, Charrondière RU, van Kappel AL, Ocke MC, Welch A, Lagiou A, van Liere M, Agudo A, Pala V, Brandstetter B, Andren C, Stripp C, van Staveren WA & Riboli E (1999): Structure of the standardized computerized 24-hour diet recall interview used as reference method in the 22 centers participating in the EPIC project. European Prospective Investigation into Cancer and Nutrition. Comput. Methods Programs Biomed. 58, 251–266.
Slimani N, Ferrari P, Ocké M, Welch A, Boeing H, van Liere M, Pala V, Amiano P, Lagiou A, Mattisson I, Stripp C, Engeset D, Charrondière R, Buzzard M, van Staveren W & Riboli E (2000): Standardization of the 24-hour diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): general concepts and preliminary results. Eur. J. Clin. Nutr. 54, 900–917.
Strachan DP, John WH & Spector TD (2001): Concordance and interrelationship of atopic diseases and markers of allergic sensitization among adult female twins. J. Allergy Clin. Immunol. 108, 901–907.
Stulnig TM (2003): Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int. Arch. Allergy Immunol. 132, 310–321.
Trak-Fellermeier MA, Brasche S, Winklerz G, Koletzko B & Heinrich J (2004): Food and fatty acid intake and atopic disease in adults. Eur. Respir. J. 23, 575–582.
Valenzuela A, von Bernhardi R, Valenzuela V, Ramirez G, Alarcon R, Sanhueza J & Nieto S (2004): Supplementation of female rats with a-linolenic acid or docosahexaenoic acid leads to the same omega-6/omega-3 LC-PUFA accretion in mother tissue and in fetal and newborn brains. Ann. Nutr. Metab. 48, 28–35.
Valsta LM, Salminen I, Aro A & Mutanen M (1996): Alpha-linolenic acid in rapeseed oil partly compensates for the effect of fish restriction on plasma long chain n-3 fatty acids. Eur. J. Clin. Nutr. 50, 229–235.
von Mutius E (2000): The environmental predictors of allergic disease. J. Allergy Clin. Immunol. 105, 9–19.
von Schacky C, Fischer S & Weber PC (1985): Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J. Clin. Invest. 76, 1626–1631.
Wakai K, Okamoto K, Tamakoshi A, Lin Y, Nakayama T & Ohno Y (2001): Seasonal allergic rhinoconjunctivitis and fatty acid intake: a cross-sectional study in Japan. Ann. Epidemiol. 11, 59–64.
Winkler J & Stolzenberg H (1999): Der Sozialschichtindex im Bundesgesundheitssurvey. Gesundheitswesen 61 (S2), S178–S183.
Woods RK, Raven JM, Walters EH, Abramson MJ & Thien FCK (2004): Fatty acid levels and risk of asthma in young adults. Thorax 59, 105–110.
Wren JJ & Szczepanowska AD (1964): Chromatography of lipids in presence of an antioxidant, 4-methyl-2,6-di-tert. Butylphenol. J. Chromatog. 14, 387–404.
Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA & Calder PC (2000): Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur. J. Clin. Invest. 30, 260–274.
Yu G & Bjorksten B (1998): Polyunsaturated fatty acids in school children in relation to allergy and serum IgE levels. Pediatr. Allergy Immunol. 9, 133–138.
Yu G, Kjellman NI & Bjorksten B (1996): Phospholipid fatty acids in cord blood: family history and development of allergy. Acta Paediatr. 85, 679–683.
Acknowledgements
We acknowledge the cooperation of the study participants as well as the work of all co-workers involved in the sampling of data and biological specimens. We especially thank the physicians from the health offices in Bavaria for providing study rooms and for drawing the blood samples and Drs Weidner and Gabrio, Landesgesundheitsamt Baden-Württemberg, for IgE measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: J Linseisen was accountable for all parts of the completed manuscript before and after publication.
Contributors: SH—red blood cell analysis, analysis of data, and writing the manuscript. HS—collection and processing of data. JH and IK—significant comments on statistical analysis and interpretation of the results. GN, AN and NB—advice and consultation on data analysis and contribution to the interpretation. KG, GK and GW—fund raising and study design. JL—senior author; responsible for study design, collection and analysis of data, writing the manuscript. All authors commented on the final version.
Rights and permissions
About this article
Cite this article
Hoff, S., Seiler, H., Heinrich, J. et al. Allergic sensitisation and allergic rhinitis are associated with n-3 polyunsaturated fatty acids in the diet and in red blood cell membranes. Eur J Clin Nutr 59, 1071–1080 (2005). https://doi.org/10.1038/sj.ejcn.1602213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602213
Keywords
This article is cited by
-
Dietary meat and fat intake and prevalence of rhinoconjunctivitis in pregnant Japanese women: baseline data from the Kyushu Okinawa Maternal and Child Health Study
Nutrition Journal (2012)
-
Polyunsaturated fatty acid intake and prevalence of eczema and rhinoconjunctivitis in Japanese children: The Ryukyus Child Health Study
BMC Public Health (2011)
-
Dietary fish oil n−3 fatty acids increase regulatory cytokine production and exert anti‐inflammatory effects in two murine models of inflammation
Lipids (2006)