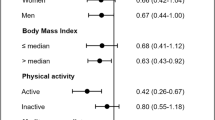
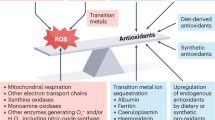
Abstract
Objective: We reviewed the bioavailability and antioxidant effects of phenols from extra virgin olive oil.
Search strategy: We searched the MEDLINE database for the years 1966–2002. To review the bioavailability of olive oil phenols, we selected animal and human studies that studied the absorption, metabolism, and urinary excretion of olive oil phenols. We also estimated the intake of the various phenols in the Mediterranean area. To review the antioxidant effects of olive oil phenols, we included human and animal studies on the effect of olive oil phenols on markers of oxidative processes in the body. We excluded studies without a proper control treatment and studies in which the antioxidant effects of phenols could not be disentangled from those of the fatty acid composition of olive oil.
Results: Bioavailability studies in humans show that the absorption of olive oil phenols is probably larger than 55–66 mol%, and that at least 5% is excreted in urine as tyrosol and hydroxytyrosol. Animal studies suggest that phenol-rich olive oil lowers oxidisability of ex vivo low-density lipoprotein (LDL) particles or lowers markers in urine of oxidative processes in the body. In five out of seven human studies, however, these effects of phenols were not found. There are no data on the phenol concentrations in plasma that are attainable by intake of olive oil. We estimated that 50 g of olive oil per day provides about 2 mg or ∼13 μmol of hydroxytyrosol-equivalents per day, and that the plasma concentration of olive oil phenols with antioxidant potential resulting from such an intake can be at most 0.06 μmol/l. This is much lower than the minimum concentrations of these phenols (50–100 μmol) required to show antioxidant activity in vitro.
Conclusion: Although phenols from olive oil seem to be well absorbed, the content of olive oil phenols with antioxidant potential in the Mediterranean diet is probably too low to produce a measurable effect on LDL oxidisability or other oxidation markers in humans. The available evidence does not suggest that consumption of phenols in the amounts provided by dietary olive oil will protect LDL against oxidative modification to any important extent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB & Kromhout D (2001): Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 55, 76–81.
Bai C, Yan X, Takenaka M, Sekiya K & Nagata T (1998): Determination of synthetic hydroxytyrosol in rat plasma by GC-MS. J. Agric. Food Chem. 46, 3998–4001.
Berry EM, Eisenberg S, Friedlander Y, Harats D, Kaufmann NA, Norman Y & Stein Y (1992): Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins—the Jerusalem Nutrition Study. II. Monounsaturated fatty acids vs carbohydrates. Am. J. Clin. Nutr. 56, 394–403.
Bonanome A, Pagnan A, Biffanti S, Opportuno A, Sorgato F, Dorella M, Maiorino M & Ursini F (1992): Effect of dietary monounsaturated and polyunsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modification. Arterioscler. Thromb. 12, 529–533.
Bonanome A, Pagnan A, Caruso D, Toia A, Xamin A, Fedeli E, Berra B, Zamburlini A, Ursini F & Galli G (2000): Evidence of postprandial absorption of olive oil phenols in humans. Nutr. Metab. Cardiovasc. Dis. 10, 111–120.
Boskou D (2000): Olive oil. World Rev. Nutr. Diet. 87, 56–77.
Carbonneau MA, Leger CL, Monnier L, Bonnet C, Michel F, Fouret G, Dedieu F & Descomps B (1997): Supplementation with wine phenolic compounds increases the antioxidant capacity of plasma and vitamin E of low-density lipoprotein without changing the lipoprotein Cu(2+)-oxidizability: possible explanation by phenolic location. Eur. J. Clin. Nutr. 51, 682–690.
Caruso D, Berra B, Giavarini F, Cortesi N, Fedeli E & Galli G (1999): Effect of virgin olive oil phenolic compounds on in vitro oxidation of human low density lipoproteins. Nutr. Metab. Cardiovasc. Dis. 9, 102–107.
Caruso D, Visioli F, Patelli R, Galli C & Galli G (2001): Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 50, 1426–1428.
Coni E, Di Benedetto R, Di Pasquale M, Masella R, Modesti D, Mattei R & Carlini EA (2000): Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 35, 45–54.
D’Angelo S, Manna C, Migliardi V, Mazzoni O, Morrica P, Capasso G, Pontoni G, Galletti P & Zappia V (2001): Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 29, 1492–1498.
Day AJ, Bao Y, Morgan MR & Williamson G (2000): Conjugation position of quercetin glucuronides and effect on biological activity. Free Radical Biol. Med. 29, 1234–1243.
DerSimonian R & Laird N (1986): Meta-analysis in clinical trials. Control. Clin. Trials. 7, 177–188.
Fito M, Gimeno E, Covas MI, Miro E, Lopez-Sabater MC, Farre M, de la Torre R & Marrugat J (2002): Postprandial and short-term effects of dietary virgin olive oil on oxidant/antioxidant status. Lipids 37, 245–251.
Food and Agricultural Organization (2000): Food Balance Sheets, 1999. Rome: FAO.
Gimeno E, Fito M, Lamuela-Raventos RM, Castellote AI, Covas M, Farre M, Torre-Boronat MC & Lopez-Sabater MC (2002): Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur. J. Clin. Nutr. 56, 114–120.
Grignaffini P, Roma P, Galli C & Catapano AL (1994): Protection of low-density lipoprotein from oxidation by 3,4-dihydroxyphenylethanol (letter). Lancet 343, 1296–1297.
Gutfinger T (1981): Polyphenols in olive oil. J. Am. Oil Chem. Soc. 58, 966–968.
Halliwell B (2000): Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc. Res. 47, 410–418.
Helsing E (1995): Traditional diets and disease patterns of the Mediterranean, circa 1960. Am. J. Clin. Nutr. 61, 1329S–1337S.
Herrera MD, Perez-Guerrero C, Marhuenda E & Ruiz-Gutierrez V (2001): Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar-Kyoto and spontaneously hypertensive rats. Br. J. Nutr. 86, 349–357.
Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ & Katan MB (1995): Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 62, 1276–1282.
Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH & Katan MB (1997): Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 418, 152–156.
Katan MB, Zock PL & Mensink RP (1995): Dietary oils, serum lipoproteins, and coronary heart disease. Am. J. Clin. Nutr. 61, 1368S–1373S.
Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F & Keys MH (1986): The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 124, 903–915.
Leenen R, Roodenburg AJ, Vissers MN, Schuurbiers JA, Van Putte KP, Wiseman SA & van de Put FH (2002): Supplementation of plasma with olive oil phenols and extracts: influence on LDL oxidation. J. Agric. Food Chem. 50, 1290–1297.
Manach C, Morand C, Crespy V, Demigne C, Texier O, Regerat F & Remesy C (1998): Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 426, 331–336.
Manna C, Galletti P, Maisto G, Cucciolla V, D'Angelo S & Zappia V (2000): Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 470, 341–344.
Masella R, Giovannini C, Vari R, Di Benedetto R, Coni E, Volpe R, Fraone N & Bucci A (2001): Effects of dietary virgin olive oil phenols on low density lipoprotein oxidation in hyperlipidemic patients. Lipids 36, 1195–1202.
Mata P, Varela O, Alonso R, Lahoz C, de OM & Badimon L (1997): Monounsaturated and polyunsaturated n-6 fatty acid-enriched diets modify LDL oxidation and decrease human coronary smooth muscle cell DNA synthesis. Arterioscler. Thromb. Vasc. Biol. 17, 2088–2095.
Miro-Casas E, Farre Albadalejo M, Covas Planells MI, Fito Colomer M, Lamuela Raventos RM & de la Torre Fornell R (2001a): Tyrosol bioavailability in humans after ingestion of virgin olive oil. Clin. Chem. 47, 341–343.
Miro-Casas E, Farre Albaladejo M, Covas MI, Rodriguez JO, Menoyo Colomer E, Lamuela Raventos RM & de la Torre R (2001b): Capillary gas chromatography-mass spectrometry quantitative determination of hydroxytyrosol and tyrosol in human urine after olive oil intake. Anal. Biochem. 294, 63–72.
Montedoro G, Servili M, Baldioli M, Selvaggini R, Miniati E & Macchioni A (1993): Simple and hydrolyzable compounds in virgin olive oil. III. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 41, 2228–2234.
Moschandreas J, Vissers MN, Wiseman S, Van Putte KP & Kafatos A (2002): Extra virgin olive oil phenols and markers of oxidation in Greek smokers: a randomized cross-over study. Eur. J. Clin. Nutr. 56, 1024–1029.
Nicolaiew N, Lemort N, Adorni L, Berra B, Montorfano G, Rapelli S, Cortesi N & Jacotot B (1998): Comparison between extra virgin olive oil and oleic acid rich sunflower oil: effects on postprandial lipemia and LDL susceptibility to oxidation. Ann. Nutr. Metab. 42, 251–260.
Ochoa-Herrera JJ, Huertas JR, Quiles JL & Mataix J (2001): Dietary oils high in oleic acid, but with different non-glyceride contents, have different effects on lipid profiles and peroxidation in rabbit hepatic mitochondria. J. Nutr. Biochem. 12, 357–364.
Ochoa JJ, Quiles JL, Ramirez-Tortosa MC, Mataix J & Huertas JR (2002): Dietary oils high in oleic acid but with different unsaponifiable fraction contents have different effects in fatty acid composition and peroxidation in rabbit LDL. Nutrition 18, 60–65.
Owen RW, Mier W, Giacosa A, Hull WE, Spiegelhalder B & Bartsch H (2000): Phenolic compounds and squalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 38, 647–659.
Papadopoulos G & Boskou D (1991): Antioxidant effect of natural phenols on olive oil. J. Am. Oil Chem. Soc. 68, 669–671.
Petroni A, Blasevich M, Salami M, Papini N, Montedoro GF & Galli C (1995): Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 78, 151–160.
Radtke J, Linseisen J & Wolfram G (1998): Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z. Ernahrungswiss. 37, 190–197.
Ramirez-Tortosa MC, Urbano G, Lopez-Jurado M, Nestares T, Gomez MC, Mir A, Ros E, Mataix J & Gil A (1999): Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J. Nutr. 129, 2177–2183.
Reaven P, Parthasarathi S, Grasse BJ, Miller E, Almazan F, Mattson FH, Khoo JC, Steinberg D & Witztum JL (1991): Feasibility of using an oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modication in humans. Am. J. Clin. Nutr. 54, 701–706.
Rice-Evans CA, Miller NJ & Paganga G (1996): Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 20, 933–956.
Ruiz-Gutierrez V, Vazquez CM & Santa-Maria C (2001): Liver lipid composition and antioxidant enzyme activities of spontaneously hypertensive rats after ingestion of dietary fats (fish, olive and high-oleic sunflower oils). Biosci. Rep. 21, 271–285.
Steinberg D & Witztum JL (2002): Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation 105, 2107–2111.
Tuck KL, Freeman MP, Hayball PJ, Stretch GL & Stupans I (2001): The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 131, 1993–1996.
Tuck KL, Hayball PJ & Stupans I (2002): Structural characterization of the metabolites of hydroxytyrosol, the principal phenolic component in olive oil, in rats. J. Agric. Food Chem. 50, 2404–2409.
Vinson JA, Jang J, Dabbagh YA, Serry MM & Cai S (1995): Plant polyphenols exhibit lipoprotein-bound antioxidant activity using an in vitro model for heart disease. J. Agric. Food Chem. 43, 2798–2799.
Visioli F, Bellomo G, Montedoro G & Galli C (1995): Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 117, 25–32.
Visioli F, Caruso D, Galli C, Viappiani S, Galli G & Sala A (2000a): Olive oils rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Commun. 278, 797–799.
Visioli F, Caruso D, Plasmati E, Patelli R, Mulinacci N, Romani A, Galli G & Galli C (2001): Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radical Res. 34, 301–305.
Visioli F & Galli C (1995): Natural antioxidants and prevention of coronary heart disease: the potential role of olive oil and its minor constituents. Nutr. Metab. Cardiovasc. Dis. 5, 306–314.
Visioli F & Galli C (1998): Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 46, 4292–4296.
Visioli F, Galli C, Bornet F, Mattei A, Patelli R, Galli G & Caruso D (2000b): Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 468, 159–160.
Visioli F, Galli C, Plasmati E, Viappiani S, Hernandez A, Colombo C & Sala A (2000c): Olive phenol hydroxytyrosol prevents passive smoking-induced oxidative stress. Circulation 102, 2169–2171.
Visioli F, Poli A & Galli C (2002): Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 22, 65–75.
Vissers MN, Zock PL, Leenen R, Roodenburg AJ, van Putte K.P.A.M. & Katan MB (2001a): Effect of consumption of phenols from olives and extra virgin olive oil on LDL oxidizability in healthy humans. Free Radical Res. 35, 619–629.
Vissers MN, Zock PL, Roodenburg AJ, Leenen R & Katan MB (2002): Apparent absorption of olive oil phenols in humans. J. Nutr. 132, 409–417.
Vissers MN, Zock PL, Wiseman SA, Meyboom S & Katan MB (2001b): Effect of phenol-rich extra virgin olive oil on markers of oxidation in healthy volunteers. Eur. J. Clin. Nutr. 55, 334–341.
Wiseman SA, Mathot JN, de Fouw N & Tijburg LB (1996): Dietary non-tocopherol antioxidants present in extra virgin olive oil increase the resistance of low density lipoproteins to oxidation in rabbits. Atherosclerosis 120, 15–23.
Wiseman SA, Tijburg LB & van de Put FH (2002): Olive oil phenolics protect LDL and spare vitamin E in the hamster. Lipids 37, 1053–1057.
Witztum JL & Steinberg D (1991): Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88, 1785–1792.
Yamamoto N, Moon JH, Tsushida T, Nagao A & Terao J (1999): Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch. Biochem. Biophys. 372, 347–354.
Acknowledgements
We thank Stella van Boom, Marjolein Hagemans, Rianne Leenen, Annet Roodenburg, Philip Rijken, Karel van Putte, and Lilian Tijburg for their information and valuable comments.
Author information
Authors and Affiliations
Contributions
Guarantor: MN Vissers.
Contributors: MV searched the Medline database, and all authors contributed to the writing of the review.
Additional information
Supported by the International Olive Oil Council and the Foundation for Nutrition and Health Research
Rights and permissions
About this article
Cite this article
Vissers, M., Zock, P. & Katan, M. Bioavailability and antioxidant effects of olive oil phenols in humans: a review. Eur J Clin Nutr 58, 955–965 (2004). https://doi.org/10.1038/sj.ejcn.1601917
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601917
Keywords
This article is cited by
-
Biomarkers of the metabolic syndrome: influence of selected foodstuffs, containing bioactive components
Phytochemistry Reviews (2018)
-
Effect of canola oil consumption on memory, synapse and neuropathology in the triple transgenic mouse model of Alzheimer’s disease
Scientific Reports (2017)
-
Beneficial effect of maternal olive oil supplementation on lipid profile and redox status in offspring of obese rats
Phytothérapie (2017)
-
In silico studies reveal the mechanisms behind the antioxidant and anti-inflammatory activities of hydroxytyrosol
Medicinal Chemistry Research (2016)
-
An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens
Environmental Science and Pollution Research (2016)