Abstract
Objective: To investigate whether the daily intake of red wine (RW) at a dose which inversely correlates with cardiovascular disease (CVD) risk modulates immune functions in healthy men.
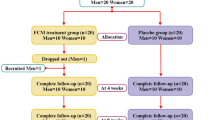
Design: Randomized single-blind trial with four intervention periods.
Setting: The Institute of Nutritional Physiology, Federal Research Centre for Nutrition, Karlsruhe, Germany.
Subjects: A total of 24 healthy males with moderate alcohol consumption patterns were recruited and all completed the study.
Interventions: Participants consumed 500 ml of RW (12% ethanol (ETOH)) or 500 ml of a 12% ETOH dilution per day for a period of 2 weeks. To control the potential effects of RW polyphenols, accordingly 500 ml/day of dealcoholized red wine (DRW) and of red grape juice (RGJ) were given. The following immune parameters were measured before beverage consumption and at 1 and 2 weeks following beverage consumption: phagocytic activity of neutrophils and monocytes, production of tumor necrosis factor-alpha (TNFα), interleukin-2 and -4, transforming growth factor-β, TNFα mRNA, lymphocyte proliferation, lytic activity of natural killer cells, and percentage of apoptotic lymphocytes.
Results: Consumption of a moderate volume of alcohol with RW and with a 12% ETOH dilution had no effect on immune functions in healthy males. Consumption of polyphenol-rich beverages (DRW and RGJ) did not affect immunity-related parameters.
Conclusions: Daily moderate consumption of alcohol and of RW for 2 weeks at doses which inversely correlate with CVD risk has no adverse effects on human immune cell functions. Polyphenol-rich beverages such as RGJ and DRW further do not suppress immune responses in healthy men.
Sponsorship: The Federal Ministry of Consumer Protection, Food, and Agriculture, Germany.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arbabi S, Garcia I, Bauer GJ & Maier RV (1999): Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J. Immunol. 162, 7441–7445.
Bagasra O, Kajdacsy-Balla A & Lischner HW (1989): Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV and of selected T-cell functions. Alcohol. Clin. Exp. Res. 13, 636–643.
Bub A, Watzl B, Heeb D, Rechkemmer G & Briviba K (2001): Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 40, 113–120.
Casalini C, Lodovici M, Briani C, Paganelli G, Remy S, Cheynier V & Dolara P (1999): Effect of complex polyphenols and tannins from red wine (WCPT) on chemically induced oxidative DNA damage in the rat. Eur. J. Nutr. 38, 190–195.
Chomczynski P & Sacchi N (1987): Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162, 156–159.
Cohen S, Tyrell DA, Russell MA, Jarvis MJ & Smith AP (1993): Smoking, alcohol consumption, and susceptibility to the common cold. Am. J. Public Health 83, 1277–1283.
Ellison RC (2002): Balancing risks and benefits of moderate drinking. Ann. NY Acad. Sci. 957, 1–6.
Exon JH, Magnuson BA, South EH & Hendrix K (1998): Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol. Immunotoxicol. 20, 173–190.
Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A & Urbano-Marquez A (1995): High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch. Int. Med. 155, 1649–1654.
Gaziano JM, Hennekens CH, Godfried SL, Sesso HD, Glynn RJ, Breslow JL & Buring JE (1999): Type of alcoholic beverage and risk of myocardial infarction. Am. J. Cardiol. 83, 52–57.
Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G & Sorensen TIA (2000): Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 133, 411–419.
Ikeda Y, Kawakami K, Sato I, Tajima S, Ito K & Nose S (1984): Effect of cianidanol (KB-53) on the activity of mouse cytotoxic T-lymphocytes. J. Pharmacobiodyn. 7, 15–20.
Laso FJ, Iglesias MC, Lopez A, Ciudad J, San Miguel JF & Orfao A (1998): Increased interleukin-12 serum levels in chronic alcoholism. J. Hepatol. 28, 771–777.
Laso FJ, Madruga JI, Giron JA, Lopez A, Ciudad J, San Miguel JF, Alvarez-Mon M & Orfao A (1997): Decreased natural killer cell cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology 26, 1096–1100.
Lodovici M, Guglielmi F, Casalini C, Meoni M, Cheynier V & Dolara P (2001): Antioxidant and radical scavenging properties in vitro of polyphenolic extracts from red wine. Eur. J. Nutr. 40, 74–77.
Lu HW, Sugahara K, Sagara Y, Masuoka N, Asaka Y, Manabe M & Kodama H (2001): Effect of three flavonoids, 5,7,3′,4′-tetrahydroxy-3-methoxy flavone, luteolin, and quercetin, on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophil. Arch. Biochem. Biophys. 393, 73–77.
Middleton E, Kandaswami C & Theoharides TC (2000): The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol. Rev. 52, 673–751.
Mohadjer C, Daniel V, Althof F & Maier H (1995): Immunological status of healthy subjects after a single intake of alcohol. Dtsch. Med. Wschr. 120, 1577–1581.
Morland B & Morland J (1984): Reduced Fc-receptor function in human monocytes exposed to ethanol in vitro. Alcohol Alcohol. 19, 211–217.
Nelson S & Kolls JK (2002): Alcohol, host defence and society. Nat. Rev. Immunol. 2, 205–209.
Ochshorn-Adelson M, Bodner G, Toraker P, Albeck H, Ho A & Kreek MJ (1994): Effects of ethanol on human natural killer cell activity: in vitro and acute, low-dose in vivo studies. Alcohol. Clin. Exp. Res. 18, 1361–1367.
O'Gorman MR (2002): Evaluation of phagocytic cell function. In Manual of Clinical Laboratory Immunology, eds NR Rose, RG Hamilton & B Detrick, pp 265–273. Washington: ASM Press.
Patel M, Keshavarzian A, Kottapalli V, Badie B, Winship D & Fields JZ (1996): Human neutrophil functions are inhibited in vitro by clinically relevant ethanol concentrations. Alcohol. Clin. Exp. Res. 20, 275–283.
Percival SS & Sims CA (2000): Wine modifies the effects of alcohol on immune cells of mice. J. Nutr. 130, 1091–1094.
Renaud SC, Guéguen R, Schenker J & d'Houtaud A (1998): Alcohol and mortality in middle-aged men from eastern France. Epidemiology 9, 184–188.
Rimm EB, Williams P, Fosher K, Criqui M & Stampfer MJ (1999): Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. Br. Med. J. 319, 1523–1528.
Roser S, Pool-Zobel BL & Rechkemmer G (2001): Contribution of apoptosis to responses in the comet assay. Mutat. Res. 497, 169–175.
Singhal PC, Patel P, Nàhàr N, Franki N, Kapasi A, Reddy K, Shah N, Nwakoby IE & Mehrotra B (1999a): Ethanol-induced neutrophil apoptosis is mediated through nitric oxide. J. Leukocyte Biol. 66, 930–936.
Singhal PC, Reddy K, Ding G, Kapasi A, Franki N, Ranjan R, Nwakoby IE & Gibbson N (1999b): Ethanol-induced macrophage apoptosis: the role of TGF-β. J. Immunol. 162, 3031–3036.
Stoltz DA, Zhang P, Nelson S, Bohm RP, Murphey-Corb M & Bagby GJ (1999): Ethanol suppression of the functional state of polymorphonuclear leukocytes obtained from uninfected and simian immunodeficiency virus infected rhesus macaques. Alcohol. Clin. Exp. Res. 23, 878–894.
Szabo G (1999): Consequences of alcohol consumption on host defence. Alcohol Alcohol. 34, 830–841.
Szabo G, Mandrekar P, Girouard L & Catalano D (1996): Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol. Clin. Exp. Res. 20, 900–907.
Takkouche B, Regueira-Méndez C, Garcia-Closas R, Figueiras A, Gestal-Otero JJ & Hernán MA (2002): Intake of wine, beer, and spirits and the risk of clinical common cold. Am. J. Epidemiol. 155, 853–858.
Verma BK, Fogarasi M & Szabo G (1993): Down-regulation of TNFα activity by acute ethanol treatment in human peripheral blood monocytes. J. Clin. Immunol. 13, 8–22.
Wang H, Cao G & Prior RL (1997): Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 45, 304–309.
Watzl B, Bub A, Blockhaus M, Herbert BM, Lührmann PM, Neuhäuser-Berthold M & Rechkemmer G (2000): Prolonged tomato juice consumption has no effect on cell-mediated immunity of well-nourished elderly men and women. J. Nutr. 30, 1719–1723.
Watzl B, Bub A, Briviba K & Rechkemmer G (2002): Acute intake of moderate amounts of red wine or alcohol has no effect on the immune system of healthy men. Eur. J. Nutr. 41, 264–270.
Watzl B, Lopez M, Shabazian M, Chen G, Colombo LL, Huang DS, Way D & Watson RR (1993): Diet and ethanol modulate immune response in young C57BL/6 mice. Alcohol. Clin. Exp. Res. 17, 623–630.
Watzl B & Watson RR (1992): Role of alcohol abuse in nutritional immunosuppression. J. Nutr. 122, 733–737.
Watzl B & Watson RR (1993): Alcohol and cytokine secretion. In Alcohol, Immunity, and Cancer, eds R Yirmiya & AN Taylor. pp 87–101. Boca Raton: CRC Press.
Acknowledgements
We gratefully acknowledge the competent technical assistance of the IEP technicians and are indebted to all study participants for contributing continuously to this study.
Author information
Authors and Affiliations
Contributions
Guarantors: B Watzl and G Rechkemmer.
Contributors: BW, AB, and GR contributed to the study design. AB was responsible for the human intervention study. BW, GP, SR, and SWB supervised the assays and analyzed the data. BW drafted the first manuscript and edited the final draft of the manuscript with contributions of all the authors.
Corresponding author
Rights and permissions
About this article
Cite this article
Watzl, B., Bub, A., Pretzer, G. et al. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur J Clin Nutr 58, 40–45 (2004). https://doi.org/10.1038/sj.ejcn.1601742
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601742
Keywords
This article is cited by
-
Effects of de-alcoholised wines with different polyphenol content on DNA oxidative damage, gene expression of peripheral lymphocytes, and haemorheology: an intervention study in post-menopausal women
European Journal of Nutrition (2011)
-
Improvement of leucocyte functions in mature and old mice after 15 and 30 weeks of diet supplementation with polyphenol-rich biscuits
European Journal of Nutrition (2011)
-
Effects of moderate beer consumption on first-line immunity of healthy adults
Journal of Physiology and Biochemistry (2007)
-
Suicide gene therapy: conversion of ethanol to acetaldehyde mediated by human beta 2 alcohol dehydrogenase
Cancer Gene Therapy (2004)