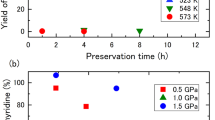
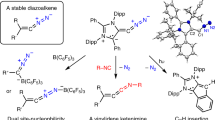
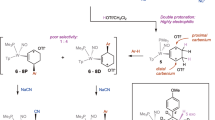
Abstract
To test the suggestion1 that the decomposition of aromatic diazo compounds might be non-ionic in mechanism, I have been investigating the decomposition of benzene-diazonium chloride in the presence of non-aqueous solvents. When suspended in an organic liquid, benzene, diazonium chloride appears to melt at about 50° C. and then immediately a violent decomposition sets in. There is great heat evolution, and, except on the small scale, the reaction tends to become explosively violent. Often hydrogen chloride is evolved, and whenever its formation has been observed, chlorobenzene has been found amongst the reaction products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Waters, J. Chem. Soc., 114 (1937).
Ber., 62, 1010, 1018 (1929); 68, 1877 (1935).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WATERS, W. Decomposition of Benzene-Diazonium Chloride. Nature 140, 466–467 (1937). https://doi.org/10.1038/140466c0
Issue Date:
DOI: https://doi.org/10.1038/140466c0
This article is cited by
-
A New Synthesis of Aromatic Arsenic Compounds
Nature (1938)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.