Abstract
[H215O]-Positron Emission Tomography (PET) was used to examine regional cerebral blood flow (rCBF) after administration of a single oral dose of the serotonin realeaser and uptake inhibitor MDMA (1.7 mg/kg) or placebo to 16 MDMA-naïve subjects. Psychological changes were assessed by psychometric rating scales. MDMA produced distributed changes in regional blood flow including increases in ventromedial frontal and occipital cortex, inferior temporal lobe and cerebellum; and decreases in the motor and somatosensory cortex, temporal lobe including left amygdala, cingulate cortex, insula and thalamus. Concomitant with these changes, subjects experienced heightened mood, increased extroversion, slight derealization and mild perceptual alterations. MDMA also produced increases in blood pressure and several side effects such as jaw clenching, lack of appetite and difficulty concentrating. These results indicate that a distributed cluster of brain areas underlie the various effects of MDMA in humans.
Similar content being viewed by others
Main
3,4-Methylenedioxymethamphetamine (MDMA) is the major component of the widely used recreational drug “Ecstasy.” In a clinical setting, MDMA produces a robust enhancement of mood and extroversion, slight derealization and a physiological response characterized by marked increases in heart rate and blood pressure (Vollenweider et al. 1998). Acute adverse effects include jaw clenching, lack of appetite, difficulty concentrating and impaired balance.
Animal studies have shown that MDMA predominantly releases serotonin via interaction with the serotonin (5-HT) transporter (Rudnick and Wall 1992; Schmidt 1987), and, to a lesser extent, also dopamine (Yamamoto and Spanos 1988). Consistent with these results, recent work in our lab demonstrated that both the psychological and physiological effects of MDMA can be attenuated by pretreatment with the selective serotonin uptake inhibitor citalopram, while the dopamine D2 antagonist haloperidol only reduced MDMA-induced positive mood (Liechti et al. 1999; Liechti et al. unpublished observations). These results suggest that the subjective and physical response to MDMA in humans is largely dependent on the enhancement of serotonergic neurotransmission.
The neurophysiological basis of the various effects of MDMA has not been examined in humans. The present study was conducted to elucidate changes in regional cerebral blood flow (rCBF) produced by administration of a single oral dose of MDMA (1.7 mg/kg) in MDMA-naïve human subjects. A recent study with the comparable 5-HT releaser d-fenfluramine in healthy subjects reported increased rCBF in medial frontal areas and decreases in the posterior temporal lobes and thalamus (Meyer et al. 1996). We hypothesized that the common serotonergic activation produced by both agents would result in overlapping changes in cerebral blood flow.
Additionally, correlations between rCBF and ratings of subjective experience were computed to obtain information on possible neural substrates of MDMA-induced changes in mood, perception and self-experience.
METHODS
Subjects
Sixteen MDMA-naïve healthy subjects (6 women and 10 men; mean age 26.0 years, S.D. = 2.5 years) without a history of drug abuse were recruited from university students and hospital staff. Subjects were healthy according to medical history, physical examination, electrocardiogram and blood analysis. A semi-structured psychiatric interview revealed no current or past psychiatric illness in any subject or their first-degree relatives. No subject had ever received psychiatric, psychotherapeutic or psychopharmacological treatment.
Written consent was obtained from all subjects after they had received written and oral descriptions of the aims of the study, the experimental procedures involved and the effects and possible risks of MDMA administration.
Design
In a double-blind design, subjects received a single oral dose of 1.7 mg/kg MDMA or placebo in randomized and counterbalanced order. The two experimental sessions were separated by at least 2 weeks. PET measurements started 75 min after drug intake, at the time of peak drug effects (Vollenweider et al. 1998). In order to standardize cognitive activity during PET scans, all subjects performed a visual Continuous Performance Test (CPT) and a corresponding control task. In five subjects, the control task was replaced by a simple resting state measurement with eyes open. On a given day, each subject received four 60-second PET scans, two during the CPT and two during the control task/resting state. Blood pressure and heart rate were monitored throughout the session. Psychometric ratings of subjective experience during the PET measurement were obtained 4 hours after drug intake, when all drug effects had subsided.
A dose of 1.7 mg/kg MDMA was chosen for the study, since it was known from a previous study to produce reliable mood effects (Vollenweider et al. 1998). Available evidence suggests that this dose is very unlikely to produce neurotoxicity or lasting functional impairments in humans (Vollenweider et al. 1999; see also Lieberman and Aghajanian 1999).
The study was approved by the Ethics Committee of the University Hospital of Psychiatriy, Zurich, and the Swiss Federal Health Office (BAG), Berne.
CPT/Control Task
We used a computerized A-X version of the CPT as described in Van Leeuwen et al. (1998). A sequence of capital letters appeared on screen and subjects’ task was to click a mouse button with the right index finger whenever the target sequence, an “A” followed by an “X,” appeared. Stimuli were presented for 150 ms in the center of the screen, between two vertical lines below and above as fixation aids (which remained constantly on-screen). The interstimulus interval was 1500 ms. Forty target sequences were presented during a total task duration of 11 minutes.
The control task was identical to the CPT, except that the target letters “A” and “X” were removed from the stimulus set. There was no attentional task for the subjects. The instruction was to simply relax and look at the screen.
Psychometric Rating Scales
The Altered States of Consciousness Questionnaire (OAV) is a visual-analog scale which measures alterations in mood, thought processes and experience of the self/ego and of the environment in drug- and non-drug-induced altered states of consciousness (Dittrich 1998; Bodmer 1989). The OAV consists of three dimensions. The first dimension, OB (“Oceanic Boundlessness”), measures derealization and depersonalization associated with a positive basic mood, and alterations in the sense of time. The second dimension, VR (“ViOsionary Restructuralization”), refers to visual illusions, hallucinations, synaesthesia and the altered experience of meaning. The third dimension, AED (“Anxious Ego Dissolution”), measures thought disorder, ego disintegration, and loss of body and thought control associated with arousal and anxiety.
The EWL Mood Questionnaire (Janke and Debus 1978) includes scales measuring activity, inactivation, extroversion, well-being, emotional excitability and anxiety. The well-being scale is composed of the two subscales “self-confidence” and “heightened mood.”
Adverse drug effects were assessed by the List of Complaints (LC). It contains 65 items describing unpleasant somatic and psychological symptoms. For the purposes of this study, the item jaw clenching was added to the list.
Statistical Analysis of Subjective and Cardiovascular Data
MANOVA with drug (placebo, MDMA) as within-subject factor was used to determine effects of MDMA on psychometric scales (OAV and EWL) and CPT performance. Two-way ANOVA with drug (placebo, MDMA) and time as factors was used to assess MDMA-induced changes in blood pressure. Post-hoc comparisons were done using Tukey's test.
Image Acquisition and Analysis
Scans were performed on a General Electric Advance PET scanner in 3D-acquisition mode. The accumulated counts over 60 seconds were taken as measure for blood flow. Mean doses of radioactivity administered per scan ranged between 400–500 MBq. The mean time between radiotracer injection and start of the scan was 40–50 seconds. Images were processed using the statistical parametric mapping software (SPM 95). Individual scans were realigned, normalized into stereotactic space (Talairach and Tournoux 1988) and smoothed with a gaussian filter (15 mm FWHM) (Friston et al. 1996). Comparisons between drug and task conditions were made on a voxel-by-voxel basis using the t-statistic and linear contrasts with opposite weights (1 and −1) for contrasting conditions. The resulting maps (SPM{t}) were transformed to the unit normal distribution (SPM{Z}). Significant main effects of drug and task as well as significant drug × task interactions were identified based on a statistical level of significance p < .05, corrected for multiple comparisons.
Correlations between rCBF and psychometrics scores were obtained for the MDMA condition using an SPM ANCOVA model that assessed covariance of psychometric scores (OAV, EWL) with mean MDMA scans (averaged over all task conditions). The level of statistical significance for regional correlations was set at p < .05, corrected for multiple comparisons.
Substance
Racemic MDMA (3,4-methylenedioxymethamphetamine) was obtained from EPROVA AG, Schaffhausen, by permission of the Swiss Federal Health Office (BAG), Department of Pharmacology and Narcotics, Berne, and was prepared as capsules (10 mg and 50 mg) at the Pharmacy of the Kantonsspital, Lucerne, Switzerland.
RESULTS
PET
Statistical parametric mapping revealed significant main effects of drug and task, but no significant task × drug interactions, indicating that task condition had no significant effect on how MDMA affected regional cerebral blood flow compared to placebo. Consistent with this, visual comparison between placebo and MDMA scans for each individual task condition (resting state, control task, CPT) showed nearly identical patterns of rCBF changes. Thus, all results provided here represent the statistical main effects of drug and task, respectively.
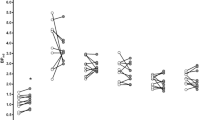
MDMA produced significant bilateral increases in rCBF in the ventromedial prefrontal cortex, the ventral anterior cingulate, the inferior temporal lobe, the medial occipital lobe, and a widespread activation of the entire cerebellum (Figure 1). MDMA decreased rCBF bilaterally in the pre- and paracentral lobule, the dorsal anterior and the posterior cingulate, the superior temporal gyrus, insula, and thalamus. One-sided decreases were found in the left amygdala and the right parahippocampal formation and uncus. Figure 1 shows statistical parametric maps of significant regional differences between MDMA and placebo. For all regions, voxels of maximal change with Talairach coordinates, Z score and level of statistical significance (corrected for multiple comparisons) are given in Table 1.
Transverse, coronal and sagittal brain sections showing significant rCBF differences between MDMA and placebo. Images are color-coded statistical parametric maps displayed according to radiological convention (left = right). Distance of planes relative to the anterior commisure are given in mm: x is the lateral distance from the midline (positive: right), y is the anteroposterior distance from the anterior commisure (positive: anterior) and z denotes the rostrocaudal distance from the bicommisural plane (positive: rostral). Upper row: MDMA-induced increases in rCBF. a = ventromedial frontal cortex (including orbitofrontal and ventral anterior cingulate cortex), b = cerebellum, c = inferior temporal cortex, d = occipital cortex. Lower row: MDMA-induced decreases in rCBF. e = superior temporal cortex, f = insula, g = Thalamus, h = pre-/paracentral cortex, k = left amygdala
Compared to the control task, the CPT produced significant changes in rCBF. CPT-induced increases (with Broadman area, Talairach x, y, z coordinates, Z score and p-values corrected for multiple testing given in parantheses) included the right medial occipital cortex (BA 18, 24, −96, 8, Z = 5.8, p < .001), the left precentral gyrus (BA 6, −50, −6, 40, Z = 5.2, p = .001), the left superior frontal gyrus (BA 9, −20, 48, 36, Z = 4.9, p = .002) and the left anterior cingulate (BA 32, −12, 28, 24, Z = 4.4, p < .02). Decreases during the CPT compared to the control task included the right medial temporal gyrus (BA 37, 54, −64, 8, Z = 7.5, p < .001), the left superior temporal gyrus (BA 22, −44, −50, 20, Z = 5.8, p < .001), the right precuneus (BA 7, 14, −48, 40, Z = 4.7, p = .004) and, at a statistical trend-level, the right medial frontal gyrus (BA 9, 10, 36, 28, Z = 3.9, p < .09).
Psychology
Predominant among the MDMA-induced subjective effects were affective changes of a generally positive nature. Compared to placebo, MDMA significantly elevated EWL scores (main effect drug (Rao R (6,10) = 8.05, p < .002). Post hoc comparisons showed significant increases for well-being (p < .001), heightened mood (p < .001), self-confidence (p < .001), extroversion (p < .001) and emotional excitability (p < .001)(Figure 2a).
MDMA-induced alterations of subjective experience during PET measurements. (A) MDMA-induced affective changes as measured by the EWL Mood Questionnaire. (B) MDMA-induced changes in dimensions of the Altered States of Consciousness Questionnaire (OAV). OB = “Oceanic Boundlessness,” VR = “Visionary Restructuralization” and AED = “Anxious Ego Dissolution.” *p < .05, **p < .01, ***p < .001. Whiskers represent Standard Errors
MDMA also produced mild derealization and depersonalization, as reflected by significantly increased OAV scores (main effect of drug Rao R (3,13) = 11.56, p < .001). Post hoc comparisons revealed significant increases for OB (“Oceanic Boundlessness”; p < .001), VR (“Visionary Restructuralization”; p < .001) and AED (“Anxious Ego Dissolution”; p = .02) (Figure 2b). The increase in OB scores was due to a prominent increase in items for positive basic mood and moderate increases in items for derealization and depersonalization. Although VR scores were elevated, none of the subjects reported hallucinations, whereas visual illusions and an intensification of tactile awareness were experienced frequently. Increased AED scores were due to thought disorder and first signs of loss of body control.
Correlations between Psychometric Scores and rCBF
No correlations reached statistical significance when corrected for multiple comparisons. For exploratory purposes, it was decided to lower the level of significance to p < .001, uncorrected for multiple comparisons. Correlations surviving this statistical threshold are given below (with Broadman area, Talairach x, y, z coordinates and Z score given in parantheses). Heightened mood correlated positively with CBF in the right parietal cortex (BA 7, 18, −58, 40, Z = 3.9) and negatively with CBF in the right caudate nucleus (−, 18, 20, 0, Z = 3.4). Oceanic Boundlessness (OB) correlated positively with CBF in the right lateral prefrtontal cortex (BA 10, 40, 50, 4, Z = 3.7), the right supramarginal gyrus (BA 40, 54, −56, 32, Z = 3.4) and the right lingual/fusiform gyrus (BA 19, 26, −66, 0, Z = 3.4). Anxious Ego Dissolution (AED) correlated positively with CBF in the left amygdala (−, −22, −8, −12, Z = 3.3), the left superior temporal gyrus (BA 22, −56, −38, 20, Z = 3.3) and negatively with CBF in the right inferior temporal/fusiform gyrus (BA 20, 40, −22, −28, Z = 3.5). EWL Extroversion correlated positively with CBF in the left precuneus (BA 7, −8, −50, 56, Z=3.5).
For the present study, these results should be regarded as purely descriptive. They may, however, serve to generate anatomically constrained hypotheses for future studies.
Cardiovascular and Adverse Effects
Blood pressure (BP) measurements were temporally averaged into three time intervals: pre-drug (ranging from 2 h before up to drug administration), 0–75 min post drug administration and 75–150 min post drug administration. Each interval contained the average of about four separate BP measurements.
MDMA moderately, but significantly, raised systolic and diastolic BP, both compared to pre-drug levels and to placebo levels. There were significant main effects of drug for systolic (F(1,13) = 17.33, p < .001) and diastolic (F(1,13) = 10.45, p < .001) BP as well as significant drug × time interactions for both systolic (F(2,26) = 15.58, p < .001) and diastolic (F(2,26) = 15.27, p < .001) BP. MDMA raised systolic BP from 120.0 ± 10.8 mm Hg pre-drug to 129.2 ± 19.6 mmHg at 0–75 min (p < .001; post hoc) and to 136.2 ± 13.9 mmHg at 75–150 min post-drug (p < .001; post hoc). Placebo levels at 0–75 min (118.3 ± 9.0 mmHg) and 75–150 min (118.3 ± 10.6 mmHg) were significantly lower than those after MDMA for the same time intervals (p < .001 for both comparisons).
MDMA also increased diastolic BP from 73.4 ± 8.4 mmHg pre-drug to 77.3 ± 12.0 mmHg at 0–75 min (p < .001; post hoc) and to 80.9 ± 10.6 mmHg at 75–150 min after drug administration (p < .001; post hoc). Placebo levels at 0–75 min (71.9 ± 7.0 mmHg) and 75–150 min (71.5 ± 8.8 mmHg) were significantly lower than those after MDMA for the same time intervals (p < .001 for both comparisons).
Consistent with our previous report (Vollenweider et al. 1998), MDMA produced a number of side effects, which, however, were not experienced with great discomfort by most subjects. Most frequently reported were jaw clenching (64% during MDMA vs. 0% during placebo), lack of appetite (63% vs. 6%), sweating (50% vs. 0%), sensitivity to cold (50% vs. 6%), dry mouth/thirst (50% vs. 6%), palpitations (38% vs. 0%) and difficulty concentrating (50% vs. 31%).
CPT Performance
There was a statistical trend for a slightly greater number of errors made in the CPT during MDMA compared to placebo (p < .06). Errors increased from 0.6 (0.15%) under placebo to 1.2 (0.3%) under MDMA. There was also a trend-level decrease (p < .09) in the number of correct responses to the target letters during MDMA. Correct responses droppped from 39.6 (99%) under placebo to 39.2 (97.9%) under MDMA.
DISCUSSION
In this study, a single oral dose of MDMA produced distributed rCBF decreases in limbic, paralimbic, central frontal and temporal areas and increases in the prefrontal, inferior temporal and cerebellar cortex in human subjects. These changes were paralleled by pronounced mood enhancement, increased extroversion, slight anxious ego dissolution, and a mild intensification of sensory perception. Cardiovascular monitoring confirmed our previous findings (Vollenweider et al. 1998) that MDMA increases blood pressure. Likewise, adverse effects were similar as reported previously and included jaw clenching, anorexia and difficulty concentrating.
It can be assumed that the observed changes in brain activity reflect several (if not all) of these psychological and physical alterations to a certain extent. With regard to possible neural substrates for MDMA-induced mood enhancement, the observed decrease in left amygdalar CBF in subjects experiencing an overall pleasurable mood state may be relevant. A decrease in left amygdala rCBF has previously been found to be correlated with feelings of euphoria during psychopharmacological stimulation in healthy subjects (Ketter et al. 1996). Furthermore, a number of functional imaging studies reported a positive correlation or association of left amygdalar activity with anxiety or sadness (Schneider et al. 1995; Ketter et al. 1996; Kalin et al. 1997), which is also in tentative agreement with the present, non-significant correlation between CBF in the left amygdala and anxiety-related psychometric scores. Thus, the observed decrease in left amygdalar flow after MDMA could provide a possible neurophysiological substrate for MDMA-induced heightened mood. However, we did not find a correlation of scores for positive mood with the decrease in left amygdala flow. Clearly, the regulatory influence of the amygdala on mood and emotional state needs to be evaluated in further studies.
A number of other areas modulated by MDMA are also likely to play a role in its prominent emotional effects. The ventral (George et al. 1995; Ketter et al. 1996; Drevets et al. 1994; Lane et al. 1997) and dorsal (Ketter et al. 1996; Paradiso et al. 1997) anterior cingulate, thalamus (George et al. 1995), temporal lobe (George et al. 1995) and cerebellum (Volkow et al. 1991; Paradiso et al. 1997) have all been implicated in emotional processing based on funtional imaging studies using pharmacologically, film- or self-induced emotion. These regions are richly interconnected and there is growing evidence that, together with the amygdala, they form a functional network for the regulation of mood and emotion (George et al. 1995). Thus, MDMA-induced emotional enhancement may rely on modulation of these limbic and paralimbic brain structures.
However, rCBF changes in some areas may also reflect non-psychological effects of MDMA. For example, MDMA may have direct cerebrovascular effects that could uncouple cerebral blood flow from neuronal activity (as indexed e.g., by firing rate or glucose/oxygen metabolism). In fact, it has been found that MDMA, although at a higher dose (5 mg/kg) than the one presently used, led to marked hyperperfusion in the rat frontal and parietal cortex without a concomitant change in glucose consumption (Kelly et al. 1994). If we assume such an effect in our study, it could either be global or local. A potential global effect would be controlled for by the applied correction for global variance. However, a local cerebrovascular effect of MDMA could not be controlled for statistically and cannot be excluded in our study.
Some of the prominent rCBF changes induced by MDMA are not immediately explained. The strong and widespread decrease in cortical somatosensory and motor areas may be related to the altered tactile awareness under MDMA. Increased flow in the visual cortex could be attributable to the mild visual alterations reported by MDMA subjects. However, there is no correlation between VR scores and CBF in this area to support such a view.
Recent evidence from our lab indicates that serotonin is mainly involved in the psychological and physiological effects of MDMA in humans, while dopamine D2 action plays a less important role (Liechti et al. 1999; Liechti et al. unpublished observations). These data suggest that 5-HT may also be the main neurotransmitter system involved in mediating the CBF effects of MDMA, at least to the extent that they are neurotransmitter effects. This view is consistent with results from a human [H215O]-PET study with the 5-HT releaser and uptake inhibitor d-fenfluramine that produced changes overlapping with those seen in the present study, particularly rCBF increases in the ventromedial frontal cortex and decreases in the temporal lobes and thalamus (Meyer et al. 1996). A further PET study in humans using the MDMA congener MDE also found some changes similar to the present study, including a prominent increase in bilateral cerebellar metabolic rate of glucose (rCMRGlu) and decreases in the precentral and superior frontal cortex (Gouzoulis-Mayfrank et al. 1999). These common changes may reflect a common serotonergic activation. However, there were also substantial non-overlapping changes in brain activity between MDMA and these other 5-HT agonists. Generally, MDMA produced a more widespread modulation of brain activity. These differences may be in part due to different methodologies, but may also reflect differential effects on receptors and neurotransmitters such as dopamine, norepinephrine and acetylcholine.
In conclusion, our study demonstrates that distributed rCBF changes in cortical and limbic/paralimbic structures parallel the various psychological and somatic effects of the 5-HT releaser MDMA in humans. MDMA may provide a useful tool to study serotonergic mechanisms of mood regulation in humans.
References
Bodmer I . (1989): Konstruktion des Fragebogens OAV zur quantitativen Erfassung aussergewöhnlicher Bewusstseinszustände. Zurich, Psychologisches Institut der Universität Zürich
Dittrich A . (1998): The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiat 31: 80–84
Drevets WC, Videen TO, Snyder AZ, MacLeod AK, Raichle ME . (1994): Regional cerebral blood flow changes during anticipatory anxiety. Soc Neurosci Abstra 20: 368
Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RJS . (1996): Spatial registration and normalization of images. Hum Brain Mapping 2: 165–189
George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM . (1995): Brain activity during transient sadness and hapiness in healthy women. Am J Psychiatry 152: 341–351
Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar K-A, Hermle L, Büll U, Sass H . (1999): Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. Neuropsychopharmacology 20: 565–581
Janke W, Debus G . (1978): Die Eigenschaftswörterliste (EWL-K) - Ein Verfahren zur Erfassung der Befindlichkeit. Göttingen, Hogrefe
Kalin NH, Davidson RJ, Irwin W, Warner G, Orendi JL, Sutton SK, Mock BJ, Sorenson JA, Lowe M, Turski PA . (1997): Functional magnetic resonance imaging studies of emotional processing in normal and depressed 32–39
Kelly PA, Ritchie IM, Sangra M, Cursham MJ, Dickson EM, Kelly B, Neilson FP, Reidy MJ, Stevens MC . (1994): Hyperaemia in rat neocortex produced by acute exposure to methylenedioxymethamphetamine. Brain Res 665: 315–318
Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, Willis MW, Herscovitch P, Post RM . (1996): Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 53: 59–69
Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ . (1997): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933
Lieberman JA, Aghajanian GK . (1999): Caveat emptor: Researchers beware. Neuropsychopharmacology 21: 471–473
Liechti ME, Baumann C, Gamma A, Vollenweider FX . (1999): Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 22 (5)): 513–521
Meyer JH, Kapur S, Wilson AA, DaSilva JN, Houle S, Brown GM . (1996): Neuromodulation of frontal and temporal cortex by intravenous d-fenfluramine: An [15O]H2O_PET study in humans. Neurosci Lett 207: 25–28
Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Watkins GL, Boles Ponto LL, Hichwa RD . (1997): Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 154: 384–389
Rudnick G, Wall SC . (1992): The molecular mechanism of “ecstasy” [3,4-methylenedioxymethamphetamine (MDMA)]: Serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci 89: 1817–1821
Schmidt CJ . (1987): Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 240: 240–247
Schneider F, Gur RE, Mozley LH, Smith RJ, Mozley PD, Censits DM, Alavi A, Gur RC . (1995): Mood effects on limbic blood flow correlate with emotional self-rating: A PET study with oxygen-15 labeled water. Psychiatry Res Neuroimaging 61: 265–283
Talairach J, Tournoux P . (1988): Co-planar stereotaxic atlas of the human brain. Stuttgart, Thieme
Van Leeuwen TH, Brandeis D, Overtoom KE, Pascual-Marqui RD, Van't Klooster B, Rothenberger A, Sergeant JA, Steinhausen HC . (1998): The continuous performance test revisited with neuroelectric brain mapping: Impaired orienting in children with attention deficits. Behav Brain Res 94: 97–110
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Ivanovic M, Hollister L . (1991): Cerebellar metabolic activation by delta-9-tetrahydro-cannabinol in human brain: A study with positron emission tomography and 18F- 2-fluoro-2-deoxyglucose. Psychiatry Res 40: 69–78
Vollenweider FX, Gamma A, Liechti ME, Huber Th . (1999): Is a single dose MDMA harmless? Neuropsychopharmacology 21: 598–600
Vollenweider FX, Gamma A, Liechti ME, Huber Th . (1998): Psychological and cardiovascular effects and short-term sequelae of MDMA (“Ecstasy”) on MDMA-naive healthy volunteers. Neuropsychopharmacology 19: 241–251
Yamamoto BK, Spanos LJ . (1988): The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol 148: 195–203
Acknowledgements
This research was supported by the UBS Science Foundation Switzerland (ANGS-MRY) and the Heffter Research Institute (HRI 41-101.98), USA. The authors thank G.K. von Schulthess for the use of the PET infrastructure, D. Brandeis for providing the Continuous Performance Test and M. Liechti for critical comments on the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gamma, A., Buck, A., Berthold, T. et al. 3,4-Methylenedioxymethamphetamine (MDMA) Modulates Cortical and Limbic Brain Activity as Measured by [H215O]-PET in Healthy Humans. Neuropsychopharmacol 23, 388–395 (2000). https://doi.org/10.1016/S0893-133X(00)00130-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(00)00130-5
Keywords
This article is cited by
-
MDMA-assisted psychotherapy for PTSD in adolescents: rationale, potential, risks, and considerations
European Child & Adolescent Psychiatry (2023)
-
The Altered States Database: Psychometric data from a systematic literature review
Scientific Data (2022)
-
A Systematic Review of Neurocognitive Effects of Subanesthetic Doses of Intravenous Ketamine in Major Depressive Disorder, Post-Traumatic Stress Disorder, and Healthy Population
Clinical Drug Investigation (2022)
-
MDMA-assisted psychotherapy for people diagnosed with treatment-resistant PTSD: what it is and what it isn’t
Annals of General Psychiatry (2020)
-
Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials
Psychopharmacology (2020)