Abstract
Research efforts to identify and understand the pathophysiology of schizophrenia and bipolar illness are limited by the inability to study neuronal tissue of living patients. An alternative to sampling brain tissue from living patients is to measure neuronal proteins found in cerebral spinal fluid. One such candidate protein is synaptosomal-associated protein 25kDa. Our hypothesis is that the level of this protein in cerebral spinal fluid may be a marker of neuronal pathology. Cerebral spinal fluid from headache, schizophrenic, bipolar, and control subjects was used to measure the SNAP-25 level by quantitative dot blotting. Schizophrenic subjects had significantly elevated levels of SNAP-25 as compared to headache and control subjects. However, there was no significant difference between the bipolar group and schizophrenic or control groups. This study reports on a potentially useful clinical marker in schizophrenia, and the presence of elevated cerebral spinal fluid SNAP-25 may indicate alterations in neuronal functioning.
Similar content being viewed by others
Main
In the past 20 years, scientists have conducted extensive research to identify the pathology and etiology of schizophrenia and bipolar illness. In schizophrenic subjects, neuroimaging and functional analysis has identified abnormalities in various brain structures (Nasrallah 1990). Neuropathological studies of schizophrenics have revealed brain changes ranging from loss of gray matter to disorganization of the normal neuronal architecture (Bloom 1993). More recently, changes in vesicular- and growth- associated proteins have been identified in post-mortem brains of schizophrenics (Barbeau et al. 1995; Browning et al. 1993; Eastwood et al. 1995; Perrone-Bizzozero et al. 1996). Based on the accumulated clinical and laboratory information, investigators have developed two models to explain the pathophysiology of schizophrenia.
The neurodegeneration hypothesis proposes that schizophrenia is the result of degeneration or loss of neurons, similar to Alzheimer's disease. This hypothesis dates to 1919, when schizophrenia was referred to as “dementia praecox” (Kraepelin 1919). However, post-mortem studies have not consistently identified evidence of abnormal neurodegeneration in schizophrenic brains (Arnold et al. 1991).
An alternative hypothesis states that the pathology of schizophrenia arises during embryonic and fetal developmental (Bloom 1993; Saugstad 1989). In this hypothesis, Weinberger (Weinberger 1987) proposes that a static brain lesion is established early in development and that neurodegeneration is not a major factor in the adult pathology. As the brain matures, it compensates for the lesion until the adaptations are overwhelmed. When the brain adaptions are insufficient to compensate, the final groups of behavioral symptoms are collectively described as schizophrenia. As in the neurodegenerative hypothesis, supporting data are circumstantial (Raedler et al. 1998).
In bipolar illness, less is known about brain pathology than with schizophrenia. Consistent findings include mood state-dependent changes in erythrocyte Na-K-ATPase activity (Looney and el-Mallakh 1997) and an increased risk for structural brain abnormalities (Altshuler et al. 1995). Both types of studies may indicate subtle neuronal damage. A question raised by both schizophrenia and bipolar studies is whether there is ongoing neuronal damage or degeneration.
The direct approach to test the hypothesis that schizophrenia and bipolar illness are not associated with ongoing neurodegeneration is to study neuronal tissue from living patients. However, moral and ethical restrictions eliminate this type of study. One way to bypass this limitation is to study a protein that has been demonstrated to be involved with the adult post-mortem pathology and can be measured in living subjects. Synaptosomal-associated protein 25kDa (SNAP-25) is a candidate protein for this type of study. We recently reported identifying this protein in human cerebral spinal fluid (CSF)(Thompson et al. 1998a), and SNAP-25 belongs to an important class of proteins involved with regulated neurotransmitter vesicle trafficking (Scheller 1995; Sollner et al. 1993). SNAP-25 is found primarily in the central nervous system and is a T-SNARE (Jacobsson et al. 1996; Roth and Burgoune 1997; Oyler et al. 1989). In human post-mortem schizophrenic cortex (Thompson et al. 1998b) the SNAP-25 levels are altered, and in an animal model of neuronal damage (Jorgensen et al. 1997), the level of SNAP-25 is decreased initially after a trauma.
In this study, we set out to determine if the level of SNAP-25 found in the CSF can differentiate pathological states. We hypothesize that SNAP-25 found primarily intracellularly will have an altered level in the extracellular space in pathological states. As a control, we used two different subject groups. The first were subjects with severe enough headaches to warrant a lumbar puncture but without other brain disorders. This group was included to demonstrate if nonspecificity exists for SNAP-25 level in nonseverely medically ill but highly stressed subjects. The second control group were normal subjects identified as free of medical or psychiatric illnesses.
METHODS
Subjects
All CSF was gathered under University of New Mexico Health Sciences Center Human Research Review Committee protocol 95-338. CSF from headache subjects was collected at the University of New Mexico Health Sciences Center Hospital clinical laboratory microbiology section. This fluid was taken for clinical indications and stored at 0°C. After 7 days, the fluid was declared waste and then stored at −80°C. A retrospective chart review provided the clinical history. To have the diagnosis of headache, individuals were required to have the clinical diagnosis of migraine or headache, and CSF cultures were without bacterial growth.
For schizophrenia, bipolar, and control groups demographic, medical, and psychiatric histories were obtained after informed consent. DSM-4 Axis 1 psychiatric diagnoses were determined by the Structured Clinical Interview (Spitizer et al. 1996), followed by a physical examination and standard blood tests. Severity of psychosis in the schizophrenia group was determined by the Brief Psychotic Rating Scale (Flemenbaum and Zimmermann 1973). When the screening was complete, control, stable schizophrenic, and bipolar subjects were admitted to the Clinical Research Center, and after a 12- hour fast, an Lumbar Puncture (LP) was performed between 7–9 AM. CSF was immediately frozen and stored at −80°C. The other schizophrenic and bipolar subjects had the same procedure; however, the LP was performed at the University of New Mexico Mental Health Center inpatient ward.
SNAP-25 Analysis
1–50 μl of the first 5 ml of CSF collected was added to a dot blot apparatus and aspirated onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-PTM, Millipore) (schizophrenia, bipolar, and control groups). The last CSF from the LP or tube four (Dougherty and Roth 1986) was used for headache subjects. The membrane was blocked in 10% powdered milk, incubated in 1:12,000 SMI-81 anti-SNAP-25 primary antibody (Sternberger Monoclonals) and 1:40,000 goat antimouse secondary antibody bound to horseradish peroxidase (HRP) (ZYMED Laboratories Inc.). We visualized the dots by enhanced chemiluminescence (NEN Life Science Products). Dot intensity was then determined by scanning and comparing gray-scale values (Gel-Pro Analyzer version 3.0, Media Cybernetics) to blotted, enriched SNAP-25 standard. Enriched SNAP standard was made by immunoprecipitating SNAP-25 from postnatal day 1 rat brains using SMI-81 (Sternberger Monoclonals) bound to cyanogenbromide (CNBR) activated sepharose beads (Pharmacia Inc.). We set as an internal control level a regression coefficient R2> 0.95 for enriched SNAP-25 standard. If the data from the standard curve fell below this level, the data were not used, and the blot was repeated. Linearity of enriched SNAP-25 has been previously published (Thompson et al. 1998b).
Statistics
All values of SNAP-25 were an average of triplicate dots repeated in three separate trials. Overall differences between group means were determined by one- way analysis of variance (ANOVA) with Fisher's least significant difference post hoc method.
RESULTS
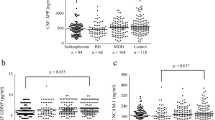
Control subjects (n = 6, age = 25.5 ± 8), were without medical diagnosis and without medication use, except for one subject who used medroxyprogesterone for birth control. Headache subjects (n = 4, age = 38.5 ± 11) had the LP, which ruled out meningitis, and the subjects did not require hospitalization, Table 1 . Bipolar illness (n = 5, age = 37 ± 13) and schizophrenic (n = 8, age = 41.6 ± 9) subjects had no medical diagnoses other than their psychiatric problems and met DSM4 criteria for their respective illnesses. All schizophrenic subjects were psychotic, and bipolar subjects were manic at the time of LP. Table 2 lists time since last known medication, years since diagnosis, and if the subjects had a substance-or alcohol-related diagnosis. Only subject 549 had a possible diagnosis of alcohol abuse binge type. This subject reported no alcohol use for at least 2 weeks before LP.
Over-all one-way analysis of variance (ANOVA) of SNAP-25 level differences between groups was significant, p = .03. There are significant differences between groups for gender (p = .001), age (p = .001) and medication use (p = .003). To determine if these factors are involved with the main finding, medication use, gender, and race were used as covariates. The significant results were maintained, adjusting for medication use covariate ANOVA p = .05, adjusting for age p = .04, adjusting for gender p = .01, and adjusting for race p = .03. In addition to covariate analysis, we eliminated the headache group, which contained only women, and reanalyzed the data. ANOVA without the headache group remained significant, p = .05.
Using Fisher's post hoc least significant difference method, the level of SNAP-25 in the schizophrenic group (4.2 ± 2.9 pg/μl) is significantly greater than controls (2.0 ± 1.6 pg/μl) and headache subjects (1.7 ± .08 pg/μl). Units are based on pg/ul of enriched SNAP-25 standard blotted on the same blot as the subjects. There was no significant difference between the schizophrenic and bipolar groups (p> .05), or between bipolar and control or headache groups, Figure 1. Within the schizophrenic group, the severity of psychosis (average BPRS = 49.2 ± 3.8) was also not associated with SNAP-25 level (p> .05).
CONCLUSION
Both preclinical and clinical studies indicate that SNAP-25 may be a relevant biological marker of mental illness, especially schizophrenia. For example, preclinically SNAP-25 expression is under hormonal influence (Lustig et al. 1993). This observation may be useful when identifying markers to study the biology associated with the increased incidence of schizophrenia in adolescence (Remschmidt 1993; Kendler et al. 1999). A second preclinical observation is that developmental SNAP-25 expression changes with in utero virus exposure (Fatemi et al. 1998). Thus, measuring SNAP-25 may offer a method to study a viral etiology of schizophrenia (Wright et al. 1995). Clinically, post-mortem studies of schizophrenics show SNAP-25 has decreased immunoreactivity in the schizophrenic hippocampus (Young et al. 1998), decreased immunoreactivity in the temporal lobe, and increased in the prefrontal area 9 (Thompson et al. 1998b), decreased immunoreactivity in the cingulate with normal levels in the frontal, temporal and parietal lobe (Gabriel et al. 1997). This information demonstrates cellular pathology at the end of life, but does not indicate if the pathology is present earlier. The data presented in this report, in contrast to the above information, supports the hypothesis that neuronal pathology is present much earlier in life in schizophrenics and possibly bipolar individuals and that the process is ongoing.
What is the mechanism responsible for the elevated level of SNAP-25 found in the CSF of schizophrenics and how does it relate to the neurodevelopmental and neurodegenerative hypothesis? SNAP-25 is involved in neurotransmitter vesicle trafficking and is found in growth cones of extending neurites during the formation of synaptic connections (Jacobsson et al. 1996). Elevated SNAP-25 level may then be associated with increased synaptic activity, increased synaptogenesis, or decreased metabolism. In support of increased synaptogenesis, are data derived from brain trauma studies that show both neuronal loss and reactive synaptogenesis. Jorgensen (Jorgensen et al. 1997) measured SNAP-25 immunoreactivity in brain homogenates of brain-contused rats. His group found significantly decreased SNAP-25 levels at 3 days post-injury followed by increasing levels to 75% of baseline at day 18. Consistent with this process are observations of Geddes et al., which demonstrated increased SNAP-25 in brain regions adjacent to deafferented areas athat had reduced SNAP (Geddes et al. 1990). Possibly, our CSF SNAP-25 level, which measures the extracellular compartment, is the mirror image of Jorgensen's and Geddes's intracellular brain homogenate SNAP-25 levels. If that is the case, then CSF SNAP-25 level may indicate subtle neuronal damage, rather than the classical damage seen in major brain trauma. However, we cannot exclude the possibility that elevated levels of SNAP-25 in our psychiatrically ill subjects may represent a combination of overactive synaptic remodeling and ongoing neurodegeneration. Studying additional specific cellular markers in the CSF will clarify the process involved.
Although the present study demonstrates elevated levels of CSF SNAP-25 in schizophrenia, several confounding factors affect our interpretation. The first is that CSF from headache subjects was not collected in a controlled research protocol; rather, it was taken from medically ill patients at different times of the day and stored for a week at 0°C. To determine if storage conditions altered SNAP-25 level, we examined repeated measurements of control SNAP-25 maintained at −80°C. After repeated freeze/thaw cycles, we observed progressively decreasing levels of immunoreactive SNAP-25 (data not shown). By measuring the SNAP-25 level between the different fractions collected in the normal controls, we also determined that there is no concentration gradient for SNAP-25 in the CSF (data not shown). These observations suggest that the SNAP-25 level difference between the headache group and controls is less than reported. Other possible confounding issues are racial and gender differences and the use of medication between groups. To address the gender differences, the headache group, which contained the majority of women, was removed from the analysis, and the over-all findings remained significant. We also used race, gender, and medication use as covariants, and, again, the results remained significant. In addition, medication use was studied preclinically, and perphenazine treatment does not effect SNAP-25 immunoreactivity in rats (Fog et al. 1976; Jorgensen 1995).
As a pilot study, only a limited number of subjects were available for this report. This small sample is likely responsible for the lack of significance between the bipolar and the other groups. Expanding the present sample should provide better resolution and increased significance between normals and affected groups.
Identifying the pathology and etiology of mental illnesses are priorities in mental health research. However, current research techniques have many limitations. For example, post-mortem studies are restricted by the availability of brain donations and cannot address what is occurring earlier in life. Noninvasive study techniques such as magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), and positron emission tomography (PET) scanning are expensive and lack ligands to many relevant targets. The method reported here circumvents the need for destructive in vivo brain biopsies and adds valuable information that other types of studies cannot.
References
Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R . (1995): T2 Hyperintensities in bipolar disorder: Magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiat 152: 1139–1144
Arnold SE, Lee VM, Gur RE, Trojanowski JQ . (1991): Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proc Natl Acad Sci USA 88: 10850–10854
Barbeau D, Liang JJ, Robitaille Y, Quirion R, Srivastava LK . (1995): Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA 92: 2785–2789
Bloom FE . (1993): Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiat 50: 224–227
Browning MD, Dudek EM, Rapier JL, Leonard S, Freeman R . (1993): Significant reductions in synapsin- but not synaptophysin-specific activity in brains of some schizophrenics. Biol Psychiat 34: 529–535
Dougherty JM, Roth RM . (1986): Cerebral spinal fluid. Emerg Med Clin North Am 4: 281–297
Eastwood SL, Burnet WJ, Harrison PJ . (1995): Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience 66: 309–319
Fatemi SH, Sidwel R, Kirst D, Akhter P, Meltzer HY, Baily K, Thuras P, Sedgwick J . (1998): Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero. Brain Res 800: 1–9
Flemenbaum A, Zimmermann RL . (1973): Inter- and intrarater reliability of the Brief Psychiatric Rating Scale. Psychol Rept 32: 783–792
Fog R, Pakkenberg H, Juul P, Bock E, Jorgensen OS, Andersen J . (1976): High-dose treatment of rats with perphenazine. Psychopharmacology 50: 305–307
Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P . (1997): Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Arch Gen Psychiat 54: 559–566
Geddes JW, Hess EJ, Hart RA, Kesslak JP, Cotman CW, Wilson MC . (1990): Lesions of hippocampal circuitry define synaptosomal-associated protein-25 (SNAP-25) as a novel presynaptic marker. Neuroscience 38: 515–525
Jacobsson GJ, Piehl F, Bark CI, Zhang X, Meister B . (1996): Differential subcellular localization of SNAP-25a and SNAP-25b RNA transcripts in spinal motoneurons and plasticity in expression after nerve injury. Mol Brain Res 37: 49–62
Jorgensen OS . (1995): SNAP-25 is the major immunoreactive component of the brain-specific D3 protein. NeuroReport 7: 73–76
Jorgensen OS, Hansen LI, Hoffman SW, Fulop Z, Stein DG . (1997): Synaptic remodeling and free radical formation after brain contusion injury in the rat. Experim Neuro 144: 326–338
Kendler KS, Tsuang MT, Hays P . (1999): Age at onset in schizophrenia. Arch Gen Psychiat 44: 881–890
Kraepelin E . (1919): Dementia Praecox and Paraphrenia. Edinburgh, Livingstone.
Looney SW, el-Mallakh RS . (1997): Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depression and Anxiety 5: 53–65
Lustig RH, Hua P, Wilson MC, Federoff HJ . (1993): Ontogeny, sex dimorphism, and neonatal sex hormone determination of synapse-associated messenger RNAs in rat brain. Mol Brain Res 20: 101–110
Nasrallah HA . (1990): Brain structure and function in schizophrenia: Evidence for fetal neurodevelopmental impairment. Curr Opin Psychiat 3: 75–78
Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC . (1989): The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109: 3039–3052
Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL . (1996): Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci USA 93: 14182–14187
Raedler TJ, Knable MB, Weinberger DR . (1998): Schizophrenia as a developmental disorder of the cerebral cortex. Curr Opin Neurobiol 8: 157–161
Remschmidt H . (1993): Childhood and adolescent schizophrenia. Curr Opin Psychiat 6: 470–479
Roth D, Burgoune RD . (1997): SNAP-25 is present in a snare complex in adrenal chromaffin cells. FEBS Lett 351: 207–210
Saugstad LF . (1989): Age at puberty and mental illness toward a neurodevelopmental etiology of Kraeplin's endogenous psychosis. Brit J Psychiat 155: 536–544
Scheller RH . (1995): Membrane trafficking in the presynaptic nerve terminal. Neuron 14: 893–897
Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE . (1993): SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324
Spitizer RL, Gibbon M, Williams BW . (1996): Structural Clinical Interview for DSM-4 Axis 1 Disorders—Patient Edition (SCID1/P, Version 2.0).
Thompson PM, Rosenberger C, Holt S, Perrone-Bizzozero NI . (1998a): Measuring presynaptic proteins in human cerebral spinal fluid. J Psych Res 32: 297–300
Thompson PM, Sower AC, Perrone-Bizzozero NI . (1998b): Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry 43: 239–243
Weinberger DR . (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiat 44: 660–669
Wright P, Takei N, Rifkin RM . (1995): Maternal influenza, obstetric complication, and schizophrenia. Am J Psychiat 152: 1714–1720
Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG . (1998): SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex 8: 261–268
Acknowledgements
PMT gratefully acknowledges grant support from NARSAD-Young Investigator Award, Seaver Research Institute and the UNM Clinical Research Center-NCRR-GCRC #MOI RR00997. The authors also acknowledge Ms Rhonda Orana for her help in obtaining the medical records and Eberhard H. Uhlenhuth, MD for his thoughtful suggestions in preparing this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thompson, P., Rosenberger, C. & Qualls, C. CSF SNAP-25 in Schizophrenia and Bipolar Illness A Pilot Study. Neuropsychopharmacol 21, 717–722 (1999). https://doi.org/10.1016/S0893-133X(99)00068-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(99)00068-8
Keywords
This article is cited by
-
Biomarkers in the cerebrospinal fluid of patients with psychotic disorders compared to healthy controls: a systematic review and meta-analysis
Molecular Psychiatry (2023)
-
Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand?
European Archives of Psychiatry and Clinical Neuroscience (2012)