Abstract
Reduced serotonergic activity has been associated with impulsive aggression in personality disordered patients in metabolite and pharmacologic challenge studies. This study used positron emission tomography to explore whether reduced serotonergic function occurs in critical brain regions such as orbital frontal and cingulate cortex that may play a role in modulating aggression. Six impulsive-aggressive patients and five healthy volunteers were evaluated for changes in regional glucose metabolism after administration of the serotonergic releasing agent d,l-fenfluramine (60 mg, p.o.) or placebo. Volunteers demonstrated increases in orbital frontal and adjacent ventral medial frontal cortex, cingulate, and inferior parietal cortex, whereas impulsive-aggressive patients showed no significant increases in glucose metabolism after fenfluramine in any region. Compared with volunteers, patients showed significantly blunted metabolic responses in orbital frontal, adjacent ventral medial and cingulate cortex, but not in inferior parietal lobe. These results are consistent with reduced serotonergic modulation of orbital frontal, ventral medial frontal, and cingulate cortex in patients with impulsive-aggressive personality disorders.
Similar content being viewed by others
Main
A dysfunctional serotonergic system has been linked to impulsive aggression in a variety of clinical and non-clinical populations, including patients with personality disorders. The precise nature of the serotonergic dysfunction, as well as the brain systems implicated in its association with impulsive aggression in personality disorders, is not yet understood and has important implications for the psychopharmacologic treatment of these disorders.
Hormone responses to pharmacologic agents (e.g., fenfluramine) that increase serotonergic activity at postsynaptic receptors have been found to be abnormal in personality disordered patients with impulsive aggression (Coccaro et al. 1989; Siever and Trestman 1993). Because impulsive aggression is unlikely to be mediated by altered hormones, more direct tests of central serotonergic responsiveness that can evaluate effects of serotonergic agents on brain regions implicated in the control of aggression are needed. One such region is the orbital prefrontal cortex, which inhibits limbic and other subcortical regions involved in the modulation of aggression.
Lesions of the frontal cortex have long been known to be associated with impulsive aggression. In a famous case, a gentle railroad worker named Phineas Gage became hostile and verbally aggressive after suffering a penetrating injury to his frontal lobe. A modern reconstruction of the lesion's location suggests that the injury was to anterior and mesial aspects of the orbital frontal cortex, as well as anterior sections of the cingulate gyrus and anterior and adjacent mesial aspects of the frontal cortex (Damasio et al. 1994). Many other case reports likewise suggest that injury to or surgical removal of regions of the frontal cortex, particularly orbitofrontal cortex, results in aggressive behaviors (Damasio et al. 1994; Volavka 1995; Heinrichs 1989; Yeudall 1977; Grafman et al. 1996).
Positron emission tomography (PET) has revealed reduced prefrontal cortical activity in patients with bipolar depression (Baxter et al. 1989), patients with personality disorders characterized by impulsive aggression (Goyer et al. 1994), alcoholic criminal offenders with a history of impulsive aggression (Linnoila et al. 1983), impulsive murderers (Raine et al. 1994), and violent psychiatric inpatients (Volkow and Tancredi 1987). Fenfluramine administration increases cortical metabolism and/or blood flow in orbital frontal cortex in normal subjects (Kapur et al. 1994; Mann et al. 1996). Reduced activation of orbital prefrontal cortex following fenfluramine might suggest reduced serotonergic modulation of this key inhibitory region, bringing us closer to a mechanism by which reduced serotonergic activity contributes to disinhibited aggression. Cingulate cortex, also innervated by the serotonergic system, is closely associated with orbital frontal cortex (Porrino and Goldman-Rakic 1996) and is involved with affective evaluation of incoming stimuli, crucial to the initiation of aggression (Damasio and van Hoesen 1983). We hypothesized that metabolic activity following fenfluramine in orbitofrontal, adjacent ventral medial and cingulate cortex would be blunted in impulsive patients compared with normal volunteers. The inferior parietal cortex, an area involved in visuospatial processing, was chosen as a contrast area. Since the subjects were resting without visual stimulation, we did not expect parietal activation.
METHOD
Subjects
Subjects were recruited from the outpatient clinics of the Bronx VA and Mt. Sinai Medical Centers and from newspaper advertisements. A physical examination, laboratory workup, chest X-ray, and EKG were performed to rule out serious medical conditions (e.g., diabetes, hypertension, heart, or CNS disease), and written informed consent was obtained according to IRB guidelines. All subjects were within 20% of ideal body weight. All but two were right handed (one patient and one normal volunteer); patients were matched to controls for handedness.
The six outpatients (mean age = 33.6, SD = 6.5; 4 males, 2 females) studied had not taken any medication for ≥2 weeks. Five of the six had never been treated with psychotropic medications, while the sixth had not had any medications for 10 months. Axis I and II diagnoses were determined at entry into the study (see Diagnostic Evaluation) and subjects were free of comorbid Axis I diagnoses including major depressive disorder, substance abuse or dependence and psychotic disorders.
Five male normal volunteers (mean age = 34.8, SD = 5.6) had no family history of Axis I disorders and no personal history of Axis I or II disorders as determined by structured clinical interview.
Diagnostic Assessment
Patients were interviewed by a rater using the Schedule for Affective Disorders and Schizophrenia (SADS; Spitzer and Endicott 1978). Selected items from the Structured Clinical Interview for DSM-IV (First et al. 1996) evaluating eating and posttraumatic stress disorders were incorporated to expand assessment of these diagnoses. The Revised Schedule for Interviewing DSM-IV Personality Disorders (Pfohl et al. 1996) assessed personality disorders based on an interview conducted by a master's level psychologist and an interview with an informant close to the patient. Interrater reliability was high (κ = 0.81 for borderline personality disorder).
All patients met criteria for “Impulse Aggression Disorder” (κ = 0.92) from the “Module for Impulse Aggression Disorder” (E. Coccaro et al. personal communication), which was adapted from DSM-IV criteria for Intermittent Explosive Disorder. To qualify, a patient must have recurrent incidents of physical or verbal aggression that a) are out of proportion to the circumstances; b) occur at least twice a week for ≥1 month; c) are not better accounted for by another Axis I disorder; and d) lead to marked distress or impairment. Past or current histories of SADS-defined bipolar I disorder and schizophrenia—confounding diagnoses that might be associated with impulsive aggression—were also exclusion criteria. Depressive symptomatology on each scan day was assessed by the Hamilton Depression Rating Scale (HDRS; Hamilton 1960) in order to evaluate its significance in correlational analyses. Impulsive-aggressive patients met criteria for borderline personality disorder (n = 4), paranoid personality disorder (n = 4) and histrionic personality disorder (n = 2).
Fenfluramine/Placebo Challenge
Three subjects received the fenfluramine/PET condition first (two patients; one normal volunteer), while eight (four patients/four normal volunteers) received the placebo/PET condition first in sessions separated by 26.9 days (SD = 18.2). All subjects had negative urine drug screens on both scan days; pregnancy tests for the two premenopausal women were also negative (fenfluramine days were menstrual days 8 and 27, respectively; prolactin results from another fenfluramine challenge protocol on day 7 of the second woman's cycle were comparable).
At 8:00 a.m., after an overnight fast, i.v. lines were inserted into a forearm vein in each of the subject's arms and kept patent by normal saline, 50–75 ml/hour. The subject was fasting and at rest, but awake, in a supine position for the remainder of the procedure, except that the subject emptied his/her bladder once after the isotope-uptake period and before the PET scan. The subject's movements were limited because even slight physical exertion can affect plasma prolactin levels.
All subjects received d,l-fenfluramine (60 mg, p.o.) or placebo tablets at 10:00 h, approximately 3.5 hours before [18F]fluorodeoxyglucose (FDG) injection (13:00–14:00 h). FDG uptake was thus when levels of fenfluramine were at their peak (4 hours after oral administration (Coccaro et al. 1996; Chase and Shoulson 1975), fenfluramine half-life = 11 hours). We chose a resting condition for the FDG uptake to minimize interactions between task performance and drug effect, as in a previous study (Mann et al. 1996); thus during the FDG uptake condition, subjects rested with eyes closed. Plasma samples were obtained for the 2 hours before the PET procedure to allow acclimatization to the i.v. catheter. Plasma levels of fenfluramine (ng/ml) and its metabolite, norfenfluramine (ng/ml), were obtained 3, 4, and 5 hours after drug administration to control for variation in absorption of oral medication and analyzed by high performance liquid chromatography (Dr. Thomas Cooper, Nathan Kline Institute). Prolactin samples were drawn at 9:45, 10:00 a.m. and 11:00 a.m. (mean of 3 samples = average baseline prolactin; ng/ml), and then hourly until 3:00 p.m.
Imaging Procedures
PET scans were obtained as described elsewhere (Haznedar et al. 1997) with a head-dedicated scanner (model 2048, GE Medical Systems, Milwaukee, WI) with measured resolution of 4.5 mm in plane (4.2–4.5 mm across 15 planes) and 5.0 mm axially. Scans were reconstructed with a blank and measured transmission scan (Hann filter, width = 3.15; typically 2–3 million counts/slice). Images were obtained in nanocuries/pixel and converted into relative metabolic rate by dividing each pixel by the mean value for the entire brain (defined by brain edge from coregistered MRI). This was done to standardize individuals’ regional patterns as widely used in comparable studies (e.g., Mann et al. 1996).
MRI acquisition methods (Shihabuddin et al. 1998) (TR = 24 ms, TE = 5 ms, flip angle = 40°, slice thickness = 1.2 mm, matrix = 256 × 256, field of view = 23 cm) were selected to provide maximal field flatness and gray/white discrimination. Images of patients and volunteers were intermixed and then screened by a neuroradiologist, who read all scans as normal. For accurate anatomical analysis, a modification of the surface-fitting method of Pelizzari et al. (1989) was used in PET/MRI coregistration.
Imaging Analyses
Analyses were designed to test a priori hypotheses with targeted comparisons for the regions of interest (ROIs) and exploratory statistical probability mapping as an empirical indicator of regions that showed metabolic alterations after fenfluramine and differential patterns between patients and volunteers.
Medial Frontal, Cingulate Gyrus, and Parietal Lobe ROIs
To test whether patients showed the hypothesized blunting effect in the inferior medial frontal and anterior cingulate gyrus, we chose these two regions and a parietal contrast region for assessment. Proportional stereotaxic atlas (Matsui and Hirano 1978) coordinates for 3 × 3 pixel square box (8.90 mm2) ROIs were used, analogous to the linear proportional method of Talairach and Tournoux (1988) but with the coregistered MRI as a template. Three axial slice levels, corresponding to slices 10, 9, and 8 in the Matsui and Hirano (1978) (approximately Talairach and Tournoux z levels −4, 8, and 16), were used to locate the medial frontal and cingulate gyrus areas; slices 2, 3, and 4 (approximately Talairach and Tournoux z levels 40, 48, and 56) were used to locate comparison areas in the parietal lobe.
Orbitofrontal and Dorsolateral Frontal ROIs
To test whether patients showed a blunted metabolic response to fenfluramine in the orbitofrontal and adjacent ventral medial frontal cortex, we chose coronal rather than axial sections because of the greater ease of reliability identifying the inferior brain margin. The most anterior coronal slice (reconstructed perpendicular to the anterior-posterior commissure line) that showed both left and right frontal lobe and the most anterior slice that showed the corpus callosum connecting the hemispheres were identified. Next, six equidistant slices between these anterior and posterior boundary slices of the frontal lobe were outlined, and an 8-pixel-thick annular band was automatically drawn and divided into 15 annular segments in each hemisphere around the circumference of each brain slice. Images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using cutoff values that were individually determined in each subject (CSF from ventricle center, white from center of fronto-orbital bundle, and gray from center of cingulate) as described elsewhere (Shihabuddin et al. 1998). For each of the above sectors, metabolic rates in PET pixels coregistered to gray were averaged, yielding a ring of cortical assessments.
Statistical Analysis
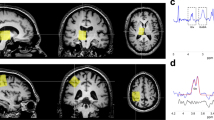
Two complementary statistical approaches, confirmatory multivariate analysis of variance (MANOVA) and exploratory statistical probability mapping, were performed. ROI-based MANOVAs combined areas of ≥80 voxels, allowing averaging across brain areas and sensitivity to widespread drug effects across cortical areas. This approach minimizes Type I errors associated with multiple statistical tests. The alternative approach, statistical probability mapping (Figures 1 and 2 ), is more sensitive than MANOVA to small areas of change and to effects in unanticipated brain areas.
Effect of fenfluramine on PET findings in impulsive patients vs. normal volunteers. A statistical probability map of relative glucose metabolic rate t-tests comparing saline minus fenfluramine condition differences is presented. Area in blue is largest contiguous patch of pixels reaching the criterion of p < .05, two-tailed for patients having a lesser metabolic change in response to fenfluramine than normal volunteers. This is the only area reaching the statistical criterion of p < .05 on the basis of statistical resampling.
Lateral surface view of brain from surface rendering of MRI with statistical probability mapping of patient vs. normal differences in fenfluramine response. T-tests (independent group tests comparing relative metabolic rate in patients vs. normal subjects: fenfluramine minus placebo condition) were carried out on rows of voxel groups, each containing about 80 PET voxels, about 2.5 FWHM (full-width half-maximum). Areas portrayed in light blue and purple are above the p < .05 threshold for patient fenfluramine increase < normal fenfluramine increase.
No data point in this report was confirmed as a statistical outlier.
MANOVA
A five-way repeated measures MANOVA was done with independent groups (patients, volunteers) and repeated measures for medication condition, hemisphere (left, right), region (cingulate, medial frontal, parietal) and level (superior, middle, inferior). All significant (p < .05) interactions involving group and drug conditions are reported. This procedure provides a single F-test of the main hypothesis that patients differ from volunteers in their glucose metabolic pattern in response to fenfluramine in different structures (diagnostic group×medication condition×region interaction). It also provides a single F-test of asymmetrical blunting. For the coronal prefrontal cortex, the MANOVA included independent groups (patients, volunteers) and repeated measures for medication condition, hemisphere (left, right), region (coronal peel sectors 1–16), and anteroposterior slice position (1–6).
Statistical Probability Mapping (SPM)
Coregistered axial PET/MRI data from each subject were morphed to the average brain contour using 500 edge points and 9 midline points placed on the MRI. Difference images (fenfluramine minus placebo) were formed, and unpaired t-tests compared patients with volunteers; the threshold of significance was established through a resampling method (Haznedar et al. 1997). The t-test manipulation is like that of Friston (1995) but uses an image standardization method utilizing visually identified anatomical landmarks and empirically derived tests of significance based on concurrent data.
For the entire cortical surface, PET data from each axial slice were divided into 50 equi-angular annular segments (each containing ˜80 pixels) for each hemisphere. T-tests compared drug-minus-placebo values in the two groups.
RESULTS
Effects of Fenfluramine in Medial Frontal, Cingulate, and Parieto-Occipital Cortex
Impulsive-aggressive patients showed less metabolic activation after fenfluramine than normal volunteers; this difference was statistically significant in the ventral medial frontal region (Table 1), right middle and left upper cingulate gyrus, and right superior parietal lobe, but not in the inferior parietal lobule, which did not show any significant between-group difference by post-hoc t-tests. Several regions, including left inferior medial frontal and middle cingulate areas, showed significant increases in relative metabolic rate in volunteers, but no area in patients showed a statistically significant fenfluramine-related change in relative metabolic rate either by follow-up ANOVA or post-hoc t-test. The occipital areas (Table 1; middle and lower ROIs) and more dorsal medial frontal areas showed no significant fenfluramine-vs.-placebo effects in either group; there were also no significant between-group differences in these areas.
Effect of Fenfluramine in Orbital and Dorsolateral Frontal Cortex
Patients had significantly smaller (blunted) increases in metabolic rate than volunteers in the left lateral orbital and right dorsolateral cortex (Table 2). Volunteers had significant post-fenfluramine increases in relative metabolic rate that were widely distributed across the prefrontal cortical surface. The drug-induced increase was greatest in left orbitofrontal and right hemisphere lateral areas (Table 2). In volunteers, increases with fenfluramine of 0.06 were found for both right and left entire frontal lobes, while in patients the increase was only 0.02 for the right hemisphere (one-third of the normal increase) and only 0.04 for the left hemisphere (ANOVA; Table 2). Although the metabolic response to fenfluramine was smaller in patients than volunteers, followup ANOVA (Table 2) confirmed a significant fenfluramine effect in the patients, consistent with a blunted rather than merely absent response. These ANOVAs were consistent with results from SPM mapping (Figures 1 and 2). On SPM, these differences were confined to the frontal cortex and were not identified in other regions including the striatum and thalamus.
Patient-Normal Comparisons at Baseline
No significant baseline differences in relative metabolic rate were found when placebo-day scans were compared in impulsive-aggressive patients and normal volunteers. For the MANOVA performed on the medial cortical ROIs, the region × hemisphere × group simple interaction showed a trend-level result (BMDP 4V, F = 3.62, df = 4.6, p = .07). For the coronal peel data for the prefrontal cortex, the region × hemisphere × position interaction was nonsignificant (F = 2.75, df = 5,5, p = .14). Studies of larger numbers of subjects may allow the detection of significant baseline differences in relative metabolic rate between patients and volunteers, but baseline differences appear to be far less marked than those evoked in response to fenfluramine challenge.
Effect of Depressed Mood and Lifetime Diagnosis of Depression on Fenfluramine Response
Correlation coefficients were calculated between the magnitude of fenfluramine responses (placebo-drug scores) in the medial frontal and cingulate stereotaxic regions and HDRS scores on the fenfluramine PET day and either present or lifetime diagnosis of depression. Of 36 correlations tested, a single medial frontal lobe area (left hemisphere, level 7) showed a significant correlation with depression (current diagnosis of depression). Thus, neither current level of depressive symptoms nor lifetime history of depression appeared to be a major determinant of the blunted fenfluramine response observed in impulsive-aggressive patients.
Secondary Analyses
Placebo-corrected prolactin responses to fenfluramine (defined as response on drug day minus response on placebo day) were not reduced in impulsive-aggressive patients compared with normal volunteers (Table 3). Prolactin responses did not correlate with relative metabolic rate response to fenfluramine (r = 0.58, 0.63, −0.04, 0.34, p < .05 for r > 0.811) for the left lower medial frontal, right middle cingulate, left upper cingulate and right upper parietal areas (four square-box ROIs showing a significant drug-minus-placebo response in volunteers in Table 1); the lower medial frontal and cingulate correlations, 0.58 and 0.63, would be expected to reach statistical significance with group sizes of 13 and 10, respectively). Peak fenfluramine and norfenfluramine drug levels did not differ between the patients and the normal volunteer groups (Table 3). There were also no significant correlations between fenfluramine and norfenfluramine drug levels and relative metabolic rate following fenfluramine. No significant correlation between age and the magnitude of the relative metabolic rate response to fenfluramine was found for ROIs (footnote 1; Table 1) that showed significant fenfluramine effects.
DISCUSSION
Blunted glucose metabolic responses to a serotonergic enhancing agent have been associated with impulsive aggression. This effect cannot be easily attributed to prior medication use, history of depression, current depressive symptomatology, or age. Although metabolic responses to fenfluramine have not previously been evaluated in impulsive-aggressive patients, our results are consistent with a body of literature linking reduced neuroendocrine and metabolic indices of serotonergic activity to aggression.
Fenfluramine enhances serotonergic activity by direct release of serotonin, antagonism of serotonergic reuptake, and possibly direct receptor effects (Coccaro et al. 1996). While higher doses of d,l-fenfluramine may antagonize dopamine receptors, the dosage used here probably does not directly affect the dopaminergic system (Chase and Shoulson 1975). Previous studies of fenfluramine and impulsive aggression have focused on prolactin responses to fenfluramine, which have been correlated with indices of impulsive aggression by self-report and history in personality disordered patients (Coccaro et al. 1996; Siever and Trestman 1993; O'Keane et al. 1992). These neuroendocrine findings are consistent with observations of reduced serotonergic metabolic activity in impulsive-aggressive personality disordered patients, impulsive volunteers, and criminal offenders (Åsberg et al. 1976, 1987; Brown et al. 1982; Virkkunen et al. 1987). Prolactin responses to fenfluramine, however, do not necessarily reflect brain circuits implicated in the modulation of aggression. Glucose metabolic responses to fenfluramine constitute a more direct, possibly more sensitive test of in-vivo responsiveness to serotonergic regulation. Indeed, in this study, as in a study of depressed patients (Mann et al. 1996), blunted metabolic responses to fenfluramine were not accompanied by significant differences in prolactin responses to fenfluramine. It is possible that brain metabolism is less sensitive than hormonal responses to gender effects, which may account for repeated findings of an association of blunted prolactin responses to fenfluramine and aggression in males (Coccaro et al. 1989; O'Keane et al. 1992; Moss et al. 1990), but not necessarily females (New et al. 1997).
No significant between-group differences emerged for glucose metabolic rate at baseline, although larger studies might detect such differences. However, differences in metabolic response to fenfluramine are not explained by baseline differences in global glucose metabolism; indeed, statistical analysis controlled for baseline glucose metabolic activity. Those regions found to be blunted at baseline in previous studies are similar to those regions that were reduced in their responsiveness to fenfluramine.
Earlier PET studies have also associated reduced prefrontal, particularly orbital frontal and adjacent medial frontal, cortical metabolism with aggressive behavior (Goyer et al. 1994; Raine et al. 1994; Volkow and Tancredi 1987). For example, one study (Goyer et al. 1994) found an inverse relationship between life history of “aggressive-impulsive behavior” and regional glucose metabolism in the orbital frontal cortex (Brodmann 11) and the right temporal lobe. In our study, blunted metabolic responses to fenfluramine occurred specifically in orbital and related prefrontal regions as well as cingulate cortex—regions that have been implicated in aggression—instead of globally reflecting reductions in the metabolism of all brain regions stimulated by fenfluramine in normal subjects.
While there are no previously reported fenfluramine/PET studies of impulsive-aggressive patients, PET studies of normal volunteers following d,l-fenfluramine and placebo suggest relative metabolic increases in the prefrontal cortex, particularly orbital and mesial frontal areas, and the anterior cingulate in the fenfluramine condition (Kapur et al. 1994). In a comparison of six normal volunteers and 11 untreated depressed patients, volunteers showed a significant left-sided activation of orbital, cingulate, and temporo-parietal cortex as well as a significant right-sided decrease in temporo-parietal and parietal-occipital cortex in the fenfluramine condition (Mann et al. 1996). In contrast, depressed patients demonstrated no significant global brain changes in glucose metabolism in response to fenfluramine compared with placebo under resting conditions; the patient-volunteer differences were most marked for a subgroup with suicide attempts (J. Mann personal communication), which may represent a form of self-directed aggression. In a Canadian study (Meyer et al. 1996), depressed patients did not differ from normal volunteers in their response to d-fenfluramine (0.3 mg/kg, i.v.), suggesting that the results of Mann et al. (1996) may reflect the large representation of suicidally depressed patients in their study group. In a study of normal and alcoholic subjects after meta-chlorophenylpiperazine (0.08 mg/kg, i.v.), normal subjects showed significant increases in regional glucose metabolism in orbital frontal cortex, left middle frontal gyrus, and thalamus, whereas alcoholic patients, also characterized by impulsive aggression, showed blunted or absent responses in orbitofrontal cortex (Hommer et al., 1997). Thus, these studies are consistent with the current study in suggesting that impulsive aggression, self- and other-directed, may be associated with reduced serotonergic activation of orbital and cingulate cortex.
These results are also consistent with a study of the metabolic effects of the SSRI sertraline from our laboratory (Buchsbaum et al. 1997). Patients with affective disorder doing a visual vigilance task showed increases in medial frontal and ventral cingulate areas following treatment with sertraline, not dissimilar to the results reported in the current resting condition of the study. The affective disorder patients showed a decrease in relative metabolic rate following sertraline in the dorsal cingulate (z = +35, Talairach and Tournoux 1988) but an increase in the ventral cingulate (z = −8). This dorsoventral difference in medication effect also appears in the current aggressive-impulsive data (drug-placebo entries for upper, middle and lower cingulate in Table 1), suggesting robustness of effect across attentional conditions. Conversely, tryptophan depletion lowered brain metabolism in orbitofrontal cortex in depressed patients (Bremner et al. 1997).
The exact mechanisms responsible for the blunted metabolic responses to fenfluramine have yet to be determined. Multiple serotonergic receptor subtypes including 5-HT1a, 5-HT1b, 5-HT2a, and 5-HT2c are found in cerebral cortex (Pazos et al. 1987) and may modify cerebral glucose metabolism (Freo et al. 1993; Grasby et al. 1992), either increasing or decreasing activity depending on brain region, dose, and receptor specificity of serotonergic agonist. A primate study demonstrated that aggressive behavior in the primates was inversely correlated with the number of 5-HT2 receptors in the posterior orbitofrontal cortex, the medial frontal cortex, and the amygdala, but not elsewhere in the brain. In contrast, 5-HT2 receptor number in the posterior orbital frontal cortex, the posterior temporal pole and the amygdala was directly correlated with prosocial behavior (Raleigh and Brammer 1993). These findings support the hypothesis that regionally specific effects of serotonin activity influence behavior; a high level of serotonin activity, as evidenced by 5-HT2 density, in the orbital cortex (and possibly the amygdala and temporal regions) is hypothesized to promote cooperative grooming behavior, whereas decreased levels of serotonin in the same areas, reflected by low 5-HT2 density, might promote aggressivity. However, given the multiplicity of serotonin receptors that could mediate the glucose metabolic response to fenfluramine, as well as the possibility raised by studies of serotonin metabolites that pre-synaptic availability may be reduced in impulsive-aggressive patients, further studies will be required to identify the specific serotonergic components altered in impulsive-aggressive patients. Furthermore, serotonergic neurons may have downstream effects on other brain neurotransmitter systems, for example, by activating inhibitory GABAminergic interneurons or even by modulating subcortical dopamine receptor sensitivity (Baumgarten and Lachenmayer 1985). These downstream effects are unlikely to account, however, for the proximal effects of fenfluramine on orbital frontal and cingulate cortex observed in this study. Whereas dopamine releasing activity has been reported in rats receiving higher doses of dexfenfluramine, at lower oral doses only a serotonergic effect was observed (Balcioglu and Wurtman 1998). In the d,l-fenfluramine data, no effect of fenfluramine on the metabolic rate of the striatum was observed as has been observed with agents affecting dopaminergic transmission such as neuroleptics and amphetamine (see review in Buchsbaum and Hazlett 1998), consistent with a primarily serotonergic effect of this dose of d,l-fenfluramine. Non-neuronal effects such as vascular effects of fenfluramine seem unlikely to account for the findings as fenfluramine is a vasoconstrictor (Weir et al. 1996; Mlczoch et al. 1979) which would be expected to reduce metabolic rate and, in any case, could not explain the differential regional effects of fenfluramine.
Larger samples will be required for a more definitive analysis of the relationship between glucose metabolic responses to serotonergic enhancement and impulsive-aggressive, depressive and other forms of clinical symptomatology; whereas no subject's glucose values were confirmed as statistical outliers, the effects of previous medication, order of administration or sex invite a study with a larger sample pool. While it is not currently possible to obtain a larger sample with fenfluramine, studies of responses to other serotonergic agents such as m-CPP are being carried out. Studies investigating pre-synaptic release by replacement of endogenous ligand, specific receptor subtype binding or response to agonists, and downstream effects on dopaminergic or GABAminergic indices are also indicated. The effects of serotonergic reuptake inhibitors, which have been demonstrated to ameliorate impulsive-aggressive symptoms, on the blunted metabolic response have been initiated and these studies cumulatively may point the way to more effective treatment of impulsive aggression.
References
Åsberg M, Schalling D, Träskman-Bendz L, Wägner A . (1987): Psychobiology of suicide, impulsivity, and related phenomena. In Meltzer HY (ed), Psychopharmacology: Third Generation of Progress. New York, Raven Press, pp 655–668
Åsberg M, Schalling D, Träskman-Bendz L, Thorén P . (1976): 5-HIAA in the cerebrospinal fluid: A biochemical suicide predictor. Arch Gen Psychiatry 33: 1193–1197
Balcioglu A, Wurtman R . (1998): Dexfenfluramine enhances striatal dopamine release in conscious rats via serotonergic mechanism. J Pharm and Exper Therapeutics 284 (3): 991–997
Baumgarten H, Lachenmayer L . (1985): Anatomical features and physiological properties of central serotonin neurons. Pharmacopsychiatry 18 (2): 180–187
Baxter LR, Schwartz JM, Phelps ME . (1989): Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 46: 243–250
Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS . (1997): Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry 54 (4): 364–374
Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK . (1982): Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. Am J Psychiatry 139: 741–746
Buchsbaum MS, Wu J, Siegel B, Hackett E, Trenary M, Abel L, Reynolds C . (1997): Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 41: 15–22
Buchsbaum MS, Hazlett EA . (1998): Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull 24: 343–364
Chase TH, Shoulson I . (1975): Behavior and biochemical effects of fenfluramine in patients with neurologic disease. Postgrad Med J 51: 105–109
Coccaro EF, Siever LJ, Klar H, Maurer G, Cochrane K, Cooper TB, Mohs RC, Davis KL . (1989): Serotonergic studies in patients with affective personality disorders: correlates with suicidal and impulsive-aggressive behavior. Arch Gen Psychiatry 46: 587–599
Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL . (1996): Hormonal responses to d- and d,l-fenfluramine in healthy human subjects. Neuropsychopharmacology 15: 595–607
Damasio AR, van Hoesen GW . (1983): Emotional disturbances associated with focal lesions of the limbic frontal lobe. In Heilman KM, Satz P (eds), Neuropsychology of Human Emotion. New York, Guilford Press
Damasio H, Grabowski T, Frank R, Galaburda A, Damasio AR . (1994): The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science 264: 1102–1105
First M, Spitzer R, Gibbon M, Williams J . (1996): Structured Clinical Interview for Axis I Disorders—Patient. New York, NYS Psychiatric Institute
Friston KJ . (1995): Commentary and opinion II: Statistical parametric mapping (Ontology and current issues). J Cerebral Blood Flow Metab 15: 361–370
Freo U, Ricchieri GL, Holloway HW, Soncrant TT . (1993): Time- and dose-dependent effects of the serotonergic agent quipazine on regional cerebral metabolism in rats. Brain Res 600: 249–256
Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schulz SC, Cohen RM . (1994): Positron-emission-tomography and personality disorders. Neuropsychopharmacology 10: 21–28
Grafman J, Schwab K, Warden D, Pridgen A, Brown H, Salazar A . (1996): Frontal lobe injuries, violence, and aggression: A report of the Vietnam head injury study. Neurology 46: 1231–1238
Grasby PM, Sharp T, Allen T, Kelly PAT, Grahame-Smith DG . (1992): Effects of the 5-HT1A partial agonists gepirone, ipsapirone and buspirone on local cerebral glucose utilization in the conscious rat. Psychopharmacology 106: 97–101
Hamilton M . (1960): Rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62
Heinrichs W . (1989): Frontal cerebral lesions and violent incidents in chronic neuropsychiatric patients. Biol Psychiatry 25: 174–178
Haznedar MM, Buchsbaum MS, Metzger M, Solimando A, Spiegel-Cohen J, Hollander E . (1997): Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. Am J Psychiatry 154: 1047–1050
Hommer D, Andreason PJ, Rio D, Williams W, Ruttimann UE, Momenan R, Zametkin AJ, Rawlings R, Linnoila M . (1997): Effects of m-chlorophenylpiperazine on regional brain glucose utilization: A positron emission tomographic comparison of alcoholic and control subjects. J Neurosci 17: 2796–2806
Kapur S, Meyer J, Wilson AA, Houle S, Brown GM . (1994): Modulation of cortical neuronal activity by a serotonergic agent: A PET study in humans. Brain Res 646: 292–294
Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK . (1983): Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 33: 2609–2614
Mann JJ, Malone K, Diehl DJ, Perel J, Cooper TD, Mintum MA . (1996): Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry 153: 174–182
Matsui T, Hirano A . (1978): Atlas of the Human Brain for Computerized Tomography. Tokyo, Igaku-Shoin
Meyer J, Kennedy S, Swinson R, Houle S, Brown G . (1996): Altered response to intravenous d-fenfluramine during depression using [15O]-PET. Biol Psychiatry 39: 516
Moss HB, Yao JK, Panzak GL . (1990: Serotonergic responsivity and behavioral dimensions in antisocial personality disorder. Biol Psychiatry 28: 325–338
Mlczoch J, Weir E, Reeves J, Grover R . (1979): Long term effects of the anorectic agent fenfluramine alone and in combination with aminorex on pulmonary and system circulation in the pig. Basic Res Cardiol 74 (3): 313–320
New A, Trestman RL, Benishay DS, Coccaro EF, Siever LJ . (1997): Self-injurious behavior in personality disorders. Psychiatry Res 69: 17–26
O'Keane V, Maloney E, O'Neil H, O'Connor A, Smith C, Dinan TG . (1992): Blunted prolactin response to d-fenfluramine in sociopathy: Evidence for subsensitivity of central serotonergic function. Br J Psychiatry 160: 643–646
Pazos A, Probst A, Palacios JM . (1987): Serotonin receptors in the human brain. IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience 21: 123–139
Pelizzari CA, Chen GT, Spelbring DR, Weichselbaum RR, Chen CT . (1989): Accurate three-dimensional registration of CT, PET, and/or MRI images of the brain. J Comput Assist Tomogr 13: 20–26
Pfohl B, Blum N, Zimmerman M . (1996): Structured Clinical Interview for DSM-IV. Washington, American Psychiatric Press
Porrino LJ, Goldman-Rakic PS . (1996): Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol 205: 63–76
Raleigh MI, Brammer GL . (1993): Individual differences in serotonin-2 receptors and social behavior in monkeys. Soc Neurosci Abstr 19: 592
Raine A, Buchsbaum M, Stanley J, Lottenberg S, Abel L, Stoddard J . (1994): Selective reductions in prefrontal glucose metabolism in murderers. Biol Psychiatry 36: 365–373
Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhojosula S, Singer MB, Ciaravolo TM, Luu-Hsia C . (1998): Dorsal striatal size, shape, and metabolic rate in neuroleptic-naive and previously medicated schizophrenic patients performing a verbal learning task. Arch Gen Psychiatry
Siever LJ, Trestman RL . (1993): The serotonin system and aggressive personality disorders. Int Clin Psychopharmacol 8(Suppl 2):33–39
Spitzer RL, Endicott J . (1978): Schedule for Affective Disorders and Schizophrenia. New York, NYS Psychiatric Institute
Talairach J, Tournoux P . (1988): Co-planar Stereotaxic Atlas of the Human Brain. New York, Thieme
Virkkunen M, Muutila A, Goodwin FK, Linnoila M . (1987): Cerebrospinal fluid metabolite levels in male arsonists. Arch Gen Psychiatry 44: 241–247
Volkow ND, Tancredi L . (1987): Neural substrates of violent behavior: A preliminary study with positron emission tomography. Br J Psychiatry 151: 668–673
Volavka J . (1995): Neurological, neuropsychological, and electrophysiological correlates of violent behavior, in Neurobiology of Violence. Washington, DC, American Psychiatric Press
Weir E, Reeve H, Huang J, Michelakis E, Nelson D, Hampl V, Archer S . (1996): Anorexic agents aminorex, fenfluramine, and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation 94 (9): 2216–2220
Yeudall LT . (1977): Neuropsychological assessment of forensic disorders. Can Ment Health 25: 7–15
Acknowledgements
This work was supported in part by a grant (5 MO1 RR00071) for the Mt. Sinai General Clinical Research Center from the National Institutes of Health, and grants R01-MH56140 (L.J. Siever, P.I.) and R29-MH50859 (R.L. Trestman, P.I.) by the National Institute of Mental Health.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siever, L., Buchsbaum, M., New, A. et al. d,l-fenfluramine Response in Impulsive Personality Disorder Assessed with [18F]fluorodeoxyglucose Positron Emission Tomography. Neuropsychopharmacol 20, 413–423 (1999). https://doi.org/10.1016/S0893-133X(98)00111-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00111-0
Keywords
This article is cited by
-
Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders
Brain Imaging and Behavior (2019)
-
Zur Pharmakotherapie impulsiver Aggression bei antisozialen und psychopathischen Störungen
Forensische Psychiatrie, Psychologie, Kriminologie (2018)
-
Monoamine-Sensitive Developmental Periods Impacting Adult Emotional and Cognitive Behaviors
Neuropsychopharmacology (2015)