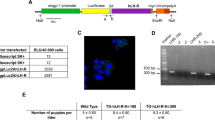
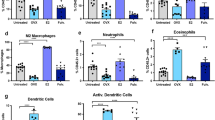
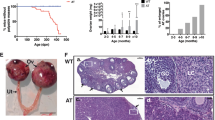
Abstract
Aneuploidy and genomic instability are common features of human cancers, including breast cancer; however, mechanisms by which such abnormalities develop are not understood. The exquisite dependence of the mammary gland on hormones for growth and development as well as hormonal contributions to breast cancer risk and progression suggest that tumorigenic mechanisms in the breast should be considered in the context of hormonal stimulation. We used transgenic mice that overexpress luteinizing hormone with subsequent ovarian hyperstimulation as a model to identify mechanisms involved in hormone-induced mammary cancer. Tumor pathology in these mice is highly variable, suggesting individual tumors undergo distinct initiating or promoting events. Supporting this notion, hormone-induced tumors display considerable chromosomal instability and aneuploidy, despite the presence of functional p53. The presence of extensive centrosome amplification in tumors and hyperplastic glands prior to tumor formation suggests that alterations in the ovarian hormonal milieu dysregulate the centrosome cycle in mammary epithelial cells, leading to aneuploidy and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Backlund MG, Trasti SL, Backlund DC, Cressman VL, Godfrey V, Koller BH . (2001). Impact of ionizing radiation and genetic background on mammary tumorigenesis in p53-deficient mice. Cancer Res 61: 6577–6582.
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG et al. (2002). Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359: 2131–2139.
Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M et al. (2001). Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92: 2247–2258.
Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH . (2003). A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res 63: 1727–1730.
Brinton LA, Schairer C, Hoover RN, Fraumeni Jr JF . (1988). Menstrual factors and risk of breast cancer. Cancer Invest 6: 245–254.
Buzdar AU, Guastalla JP, Nabholtz JM, Cuzick J, ATAC Trialists' Group. (2006). Impact of chemotherapy regimens prior to endocrine therapy: results from the ATAC (Anastrozole and Tamoxifen, alone or in Combination) trial. Cancer 107: 472–480.
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ et al. (2000). The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19: 968–988.
Cheng KC, Loeb LA . (1993). Genomic instability and tumor progression: mechanistic considerations. Adv Cancer Res 60: 121–156.
Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS . (2005). A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol 277: 443–456.
D'Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R et al. (2002). Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat 75: 25–34.
Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D et al. (1995). Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev 9: 882–895.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS et al. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221.
Duesberg P, Li R, Fabarius A, Hehlmann R . (2005). The chromosomal basis of cancer. Cell Oncol 27: 293–318.
Early Breast Cancer Trialists' Collaborative Group (1998). Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 351: 1451–1467.
Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R et al. (2002). Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res 62: 5627–5631.
Fei P, El Deiry WS . (2003). P53 and radiation responses. Oncogene 22: 5774–5783.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90: 1371–1388.
Fukasawa K, Wiener F, Vande Woude GF, Mai S . (1997). Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene 15: 1295–1302.
Goepfert TM, Adigun YE, Zhong L, Gay J, Medina D, Brinkley WR . (2002). Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res 62: 4115–4122.
Hammond SL, Ham RG, Stampfer MR . (1984). Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA 81: 5435–5439.
Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S . (1990). Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case–control study. Int J Cancer 46: 796–800.
Hundley JE, Koester SK, Troyer DA, Hilsenbeck SG, Subler MA, Windle JJ . (1997). Increased tumor proliferation and genomic instability without decreased apoptosis in MMTV-ras mice deficient in p53. Mol Cell Biol 17: 723–731.
Irwin KL, Lee NC, Peterson HB, Rubin GL, Wingo PA, Mandel MG . (1988). Hysterectomy, tubal sterilization, and the risk of breast cancer. Am J Epidemiol 127: 1192–1201.
Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla PJ et al. (2000). A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene 19: 1052–1058.
Kero J, Poutanen M, Zhang FP, Rahman N, McNicol AM, Nilson JH et al. (2000). Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J Clin Invest 105: 633–641.
Kinyamu HK, Archer TK . (2003). Estrogen receptor-dependent proteasomal degradation of the glucocorticoid receptor is coupled to an increase in MDM2 protein expression. Mol Cell Biol 23: 5867–5881.
Kuperwasser C, Hurlbut GD, Kittrell FS, Dickinson ES, Laucirica R, Medina D et al. (2000). Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumeni syndrome. Am J Pathol 157: 2151–2159.
Kvale G, Heuch I . (1988). Menstrual factors and breast cancer risk. Cancer 62: 1625–1631.
Li B, Murphy KL, Laucirica R, Kittrell F, Medina D, Rosen JM . (1998). A transgenic mouse model for mammary carcinogenesis. Oncogene 16: 997–1007.
Li B, Rosen JM, McMenamin-Balano J, Muller WJ, Perkins AS . (1997). neu/ERBB2 cooperates with p53-172H during mammary tumorigenesis in transgenic mice. Mol Cell Biol 17: 3155–3163.
Lingle WL, Salisbury JL . (1999). Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol 155: 1941–1951.
Lingle WL, Barrett SL, Negron VC, D'Assoro AB, Boeneman K, Liu W et al. (2002). Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA 99: 1978–1983.
Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL . (1998). Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci USA 95: 2950–2955.
Mann RJ, Keri RA, Nilson JH . (1999). Transgenic mice with chronically elevated luteinizing hormone are infertile due to anovulation, defects in uterine receptivity, and midgestation pregnancy failure. Endocrinology 140: 2592–2601.
Mayo LD, Donner DB . (2002). The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci 27: 462–467.
McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD . (2006). p16(INK4a) prevents centrosome dysfunction and genomic instability in primary cells. PLoS Biol 4: e51.
Miller CW, Chumakov A, Said J, Chen DL, Aslo A, Koeffler HP . (1993). Mutant p53 proteins have diverse intracellular abilities to oligomerize and activate transcription. Oncogene 8: 1815–1824.
Milliken EL, Ameduri RK, Landis MD, Behrooz A, Abdul-Karim FW, Keri RA . (2002). Ovarian hyperstimulation by LH leads to mammary gland hyperplasia and cancer predisposition in transgenic mice. Endocrinology 143: 3671–3680.
Mohammad HP, Abbud RA, Parlow AF, Lewin JS, Nilson JH . (2003). Targeted overexpression of luteinizing hormone causes ovary-dependent functional adenomas restricted to cells of the Pit-1 lineage. Endocrinology 144: 4626–4636.
Pati D, Haddad BR, Haegele A, Thompson H, Kittrell FS, Shepard A et al. (2004). Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Res 64: 5608–5616.
Pharoah PD, Day NE, Caldas C . (1999). Somatic mutations in the p53 gene and prognosis in breast cancer: a meta-analysis. Br J Cancer 80: 1968–1973.
Pihan GA, Doxsey SJ . (1999). The mitotic machinery as a source of genetic instability in cancer. Semin Cancer Biol 9: 289–302.
Pihan GA, Wallace J, Zhou Y, Doxsey SJ . (2003). Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res 63: 1398–1404.
Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD . (2001). Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 409: 633–637.
Slaughter DP, Southwick HW, Smejkal W . (1953). Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6: 963–968.
Yahagi N, Shimano H, Matsuzaka T, Najima Y, Sekiya M, Nakagawa Y et al. (2003). p53 activation in adipocytes of obese mice. J Biol Chem 278: 25395–25400.
Zell JA, Ramakrishnan R, Rathinavelu A . (2002). Regulation of mdm2 mRNA expression in human breast tumor-derived GI-101A cells. Life Sci 71: 2331–2339.
Acknowledgements
Core facilities of the Case Comprehensive Cancer Center (P30 CA43703) provided valuable services for real time RT–PCR and radiation of mice. We thank Guan-bin Lou and Ellen Barnes for their technical advice regarding metaphase spread preparation, Lindsey Mayo for thoughtful discussions regarding p53, and Kshama Mehta from Agilent Technologies Center of Excellence for performing CGH analysis and assistance with data mining. This work was funded by USAMRMC grant DAMD 17-01-1-0195, NIH Grant CA090398 (to RAK), NIH Training Grants T32GM08056 (to ELM), T32GM08803 (to MDL) and T32CA059366 (to EJ), and a predoctoral traineeship, DAMD17-03-1-0302, from the USAMRMC (to MDL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Milliken, E., Lozada, K., Johnson, E. et al. Ovarian hyperstimulation induces centrosome amplification and aneuploid mammary tumors independently of alterations in p53 in a transgenic mouse model of breast cancer. Oncogene 27, 1759–1766 (2008). https://doi.org/10.1038/sj.onc.1210815
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210815
Keywords
This article is cited by
-
Mcph1/Brit1 deficiency promotes genomic instability and tumor formation in a mouse model
Oncogene (2015)
-
Biomarker-guided sequential targeted therapies to overcome therapy resistance in rapidly evolving highly aggressive mammary tumors
Cell Research (2014)
-
Overexpression of WDR62 is associated with centrosome amplification in human ovarian cancer
Journal of Ovarian Research (2013)
-
The Relevance of Mouse Models to Understanding the Development and Progression of Human Breast Cancer
Journal of Mammary Gland Biology and Neoplasia (2008)