Abstract
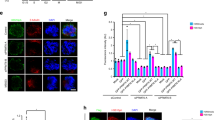
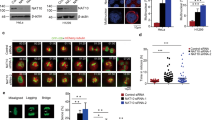
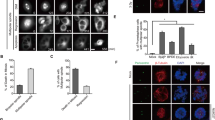
Chromosomal passenger proteins have emerged as key players in the regulation of mitosis and cytokinesis. Histone deacetylase inhibitors (HDACi) are a class of anticancer drugs that induce aberrant mitosis and can overcome the spindle assembly checkpoint. Here, we investigate the mechanism by which HDACi disrupt normal mitotic progression and checkpoint function. We demonstrate that HDACi treatment temporarily delays mitotic progression through prometaphase due to activation of the spindle assembly checkpoint. Despite failing to congress chromosomes to the metaphase plate, cells aberrantly segregate their chromosomes and exit mitosis to undergo a failed cytokinesis. We show that this premature exit from mitosis is a form of mitotic slippage. Chromosomal passenger proteins fail to accumulate at the centromere following HDACi treatment. This results in inadequate concentrations of chromosomal passenger proteins at the centromere, which are insufficient to regulate the phosphorylation of the kinetochore checkpoint component BubR1, and an inability to maintain the mitotic arrest. Thus, the centromeric accumulation of chromosomal passenger complex components is critical for regulating kinetochore but not centromeric processes, and failure of this accumulation underlies the HDACi-induced mitotic slippage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- CPC:
-
chromosomal passenger complex
- HDAC:
-
histone deacetylase
- HDACi:
-
histone deacetylase inhibitor
- SBHA:
-
suberohydroxamic acid
References
Adams RR, Maiato H, Earnshaw WC, Carmena M . (2001). Essential Roles of Drosophila Inner Centromere Protein (INCENP) and Aurora B in Histone H3 Phosphorylation, Metaphase Chromosome Alignment, Kinetochore Disjunction, and Chromosome Segregation. J Cell Biol 153: 865–880.
Aoyagi S, Archer TK . (2005). Modulating molecular chaperone Hsp90 functions through reversible acetylation. Trends Cell Biol 15: 565–567.
Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ . (2004). Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci 117: 4033–4042.
Burgess A, Ruefli A, Beamish H, Warrener R, Saunders N, Johnstone R et al. (2004). Histone deacetylase inhibitors specifically kill nonproliferating tumour cells. Oncogene 23: 6693–6701.
Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP . (2003). Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci 116: 2987–2998.
Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ . (1999). Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol 146: 941–954.
Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H et al. (2002). Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol 22: 874–885.
Delacour-Larose M, Molla A, Skoufias DA, Margolis RL, Dimitrov S . (2004). Distinct dynamics of Aurora B and Survivin during mitosis. Cell Cycle 3: 1418–1426.
Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T et al. (2003). Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol 161: 267–280.
Dowling M, Voong KR, Kim M, Keutmann MK, Harris E, Kao GD . (2005). Mitotic spindle checkpoint inactivation by trichostatin A defines a mechanism for increasing cancer cell killing by microtubule-disrupting agents. Cancer Biol Ther 4: 197–206.
Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J et al. (2005). Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438: 1116–1122.
Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R et al. (2004). Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol 166: 179–191.
Glotzer M . (2005). The molecular requirements for cytokinesis. Science 307: 1735–1739.
Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R et al. (2003). The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161: 281–294.
Hirota T, Lipp JJ, Toh BH, Peters JM . (2005). Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438: 1176–1180.
Honda R, Korner R, Nigg EA . (2003). Exploring the functional interactions between Aurora B, INCENP, and Survivin in mitosis. Mol Biol Cell 14: 3325–3341.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A et al. (2002). HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458.
Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M . (2002). The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis. Curr Biol 12: 798–812.
Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ . (2002). Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol 12: 900–905.
Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H . (2007). Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell 12: 31–43.
Klein UR, Nigg EA, Gruneberg U . (2006). Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell 17: 2547–2558.
Kops GJ, Weaver BA, Cleveland DW . (2005). On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5: 773–785.
Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM . (2004). Correcting improper chromosome–spindle attachments during cell division. Nat Cell Biol 6: 232–237.
Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T et al. (2003). Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J 22: 2934–2947.
Mackay AM, Ainsztein AM, Eckley DM, Earnshaw WC . (1998). A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J Cell Biol 140: 991–1002.
Mao Y, Desai A, Cleveland DW . (2005). Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J Cell Biol 170: 873–880.
Mollinari C, Reynaud C, Martineau-Thuillier S, Monier S, Kieffer S, Garin J et al. (2003). The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev Cell 5: 295–307.
Morrow CJ, Tighe A, Johnson VL, Scott MI, Ditchfield C, Taylor SS . (2005). Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J Cell Sci 118: 3639–3652.
Murata-Hori M, Wang YL . (2002). Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol 159: 45–53.
Qiu L, Burgess A, Fairlie DP, Leonard H, Parsons PG, Gabrielli BG . (2000). Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 11: 2069–2083.
Robbins AR, Jablonski SA, Yen TJ, Yoda K, Robey R, Bates SE et al. (2005). Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle 4: 717–726.
Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H . (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118: 187–202.
Taddei A, Maison C, Roche D, Almouzni G . (2001). Reversible disruption of pericentric heterochromatin and centromere function by inhibiting deacetylases. Nat Cell Biol 3: 114–120.
Vagnarelli P, Earnshaw WC . (2004). Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma 113: 211–222.
Verdin E, Dequiedt F, Kasler HG . (2003). Class II histone deacetylases: versatile regulators. Trends Genet 19: 286–293.
Warrener R, Beamish H, Burgess A, Waterhouse NJ, Giles N, Fairlie D et al. (2003). Tumor cell-selective cytotoxicity by targeting cell cycle checkpoints. FASEB J 17: 1550–1552.
Weaver BA, Cleveland DW . (2005). Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell 8: 7–12.
Wheatley SP, Carvalho A, Vagnarelli P, Earnshaw WC . (2001). INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr Biol 11: 886–890.
Acknowledgements
We thank WC Earnshaw, University of Edinburgh, UK, for the Borealin antibody, R Thomas, University of Queensland, for ACA serum and V Richon (Merck) for SAHA. BG is an NHMRC Senior Research Fellow. HB is a Lions Research Fellow. This work was supported by an NHMRC Australia project grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Stevens, F., Beamish, H., Warrener, R. et al. Histone deacetylase inhibitors induce mitotic slippage. Oncogene 27, 1345–1354 (2008). https://doi.org/10.1038/sj.onc.1210779
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210779
Keywords
This article is cited by
-
SIRT1 regulates mitotic catastrophe via autophagy and BubR1 signaling
Molecular and Cellular Biochemistry (2022)
-
Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe
Cell Death & Disease (2014)
-
Defective Decatenation Checkpoint Function Is a Common Feature of Melanoma
Journal of Investigative Dermatology (2014)
-
RNH1 regulation of reactive oxygen species contributes to histone deacetylase inhibitor resistance in gastric cancer cells
Oncogene (2014)
-
A potent Chk1 inhibitor is selectively cytotoxic in melanomas with high levels of replicative stress
Oncogene (2013)