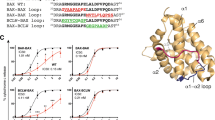
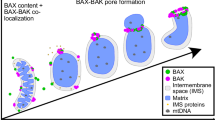
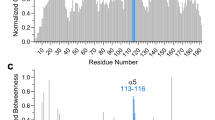
Abstract
Bax translocation from the cytosol to mitochondria culminates a key step by which this protein mediates cell death. Here, we identified two amino acids, L70 and D71, within the BH3 domain of Bax that play a critical role in regulating Bax's cytosolic vs mitochondrial distribution. Individual substitution of these amino acids with alanine resulted in Bax conformational change, oligomerization, localization to mitochondria and cell death. Further mutational analysis indicated that L70 interacts with T174, V177 and A178 of Bax's C-terminal hydrophobic segment, while the negative charge of D71 is required for maintaining Bax in its soluble monomeric state. In summary, we have identified a new regulatory site that controls Bax's subcellular distribution and activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Adams JM, Cory S . (1998). The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326.
Bowie JU, Luthy R, Eisenberg D . (1991). A method to identify protein sequences that fold into a known three-dimensional structure. Science 253: 164–170.
Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM . (2003). The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem 278: 11633–11641.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S et al. (1999). Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144: 891–901.
Eskes R, Desagher S, Antonsson B, Martinou JC . (2000). Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935.
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR . (1999). Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-XL. J Biol Chem 274: 2225–2233.
Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K et al. (1998). Regulated targeting of Bax to mitochondria. J Cell Biol 143: 207–215.
Gross A, Jockel J, Wei MC, Korsmeyer SJ . (1998). Enforced dimerization of Bax results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J 17: 3878–3885.
Hsu Y-T, Wolter KG, Youle RJ . (1997). Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Pro Natl Acad Sci USA 94: 3668–3672.
Hsu Y-T, Youle RJ . (1997). Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–13834.
Hsu Y-T, Youle RJ . (1998). Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem 273: 10777–10783.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. (2005). BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17: 525–535.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES et al. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489.
Manon S, Chaudhuri B, Guerin M . (1997). Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-XL. FEBS Lett 415: 29–32.
Mateu MG, Fersht AR . (1999). Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization. Proc Natl Acad Sci USA 96: 3595–3599.
Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Sloetjes AW, Witte TD et al. (1998). Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood 91: 2991–2997.
Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P . (2001). Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276: 18361–18374.
Nechushtan A, Smith CL, Hsu Y-T, Youle RJ . (1999). Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341.
Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ . (2001). Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol 153: 1265–1276.
Oltvai ZN, Milliman CL, Korsmeyer SJ . (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619.
Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM et al. (2005). The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA 102: 17975–17980.
Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC et al. (1997). Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275: 967–969.
Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I et al. (1998). Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391: 496–499.
Smaili SS, Hsu Y-T, Sanders KM, Russell JT, Youle RJ . (2001). Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ 8: 909–920.
Suzuki M, Youle RJ, Tjandra N . (2000). Structure of Bax. Coregulation of dimer formation and intracellular localization. Cell 103: 645–654.
Thompson CB . (1995). Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462.
Wang K, Gross A, Waksman G, Korsmeyer SJ . (1998). Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol 18: 6083–6089.
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al. (2005). Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19: 1294–1305.
Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ . (1997). Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139: 1281–1292.
Yin X-M, Oltvai ZN, Korsmeyer SJ . (1994). BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369: 321–323.
Zha H, Aime-Sempe C, Sato T, Reed JC . (1996). Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 271: 7440–7444.
Acknowledgements
We thank Dr Debbie Hansen for critical reading and Yunfei Chai for technical assistance. This research was supported by the grants RO1 NS40932 and COG RR01 5455 (MUSC animal facility) from the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on the Oncogene web site (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, H., Hou, Q., Hansen, J. et al. Complete activation of Bax by a single site mutation. Oncogene 26, 7092–7102 (2007). https://doi.org/10.1038/sj.onc.1210517
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210517
Keywords
This article is cited by
-
Progress in understanding the mechanisms of resistance to BCL-2 inhibitors
Experimental Hematology & Oncology (2022)
-
Topology of active, membrane-embedded Bax in the context of a toroidal pore
Cell Death & Differentiation (2018)
-
Targeting Bax interaction sites reveals that only homo-oligomerization sites are essential for its activation
Cell Death & Differentiation (2013)
-
Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX
Cell Research (2011)