Abstract
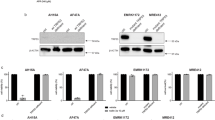
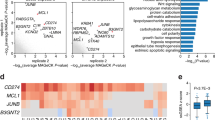
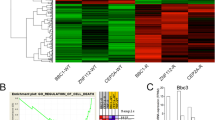
In order to define genetic determinants of primary and metastatic melanoma cell susceptibility to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), we have applied oligonucleotide microarrays to TRAIL-sensitive primary T1 cells and TRAIL-resistant metastatic G1 cells treated or not with TRAIL. T1 and G1 cells are isogenic melanoma cell subclones. We examined 22 000 spots, 4.2% of which displayed differential expression in G1 and T1 cells. Cell susceptibility to TRAIL-mediated apoptosis was found to be correlated with gene expression signatures in this model. Some of the differentially expressed genes were identified as involved in ATP-binding and signaling pathways, based on previously published data. Further analysis provided evidences that c-kit was overexpressed in G1 cells while it was absent in T1 cells. The c-kit inhibitor, imatinib, did not restore TRAIL sensitivity, excluding a role for c-kit in TRAIL resistance in G1 cells. Surprisingly, imatinib inhibited cell proliferation and TRAIL-mediated apoptosis in melanoma cells. We investigated the possible involvement of several molecules, including c-ABL, platelet-derived growth factor receptor (PDGFR), cellular FADD-like interleukin-1 α-converting enzyme-like inhibitory protein (c-FLIP)L/S, Fas-associated DD kinase, p53, p21WAF1, proteins of B-cell leukemia/lymphoma 2 (Bcl-2) family and cytochrome c. Imatinib did not modulate the expression or activation of its own targets, such as c-ABL, PDGFRα and PDGFRβ, but it did affect the expression of c-FLIPL, BCL2-associated X protein (Bax) and Bcl-2. Moreover, c-FLIPL knockdown sensitized T1 cells to TRAIL-mediated apoptosis, with a sensitivity similar to that of cells previously treated with imatinib. More notably, we found that the resistance to TRAIL in G1 cells was correlated with constitutive c-FLIPL recruitment to the DISC and the inhibition of caspase 8, 3 and 9 processing. Moreover, c-FLIPL knockdown partly restored TRAIL sensitivity in G1 cells, indicating that the expression level of c-FLIPL and its interaction with TRAIL receptor2 play a crucial role in determining TRAIL resistance in metastatic melanoma cells. Our results also show that imatinib enhances TRAIL-induced cell death independently of BH3-interacting domain death agonist translocation, in a process involving the Bax:Bcl-XL ratio, Bax:Bcl-XL/Bcl-2 translocation, cytochrome c release and caspase activation. Our data indicate that imatinib sensitizes T1 cells by directly downregulating c-FLIPL, with the use of an alternative pathway for antitumor activity, because PDGFRα is not activated in T1 cells and these cells do not express c-kit, c-ABL or PDGFRβ. Caspase cascade activation and mitochondria also play a key role in the imatinib-mediated sensitization of melanoma cells to the proapoptotic action of TRAIL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Accession codes
Abbreviations
- Bax:
-
BCL2-associated X protein
- Bcl-2:
-
B-cell leukemia/lymphoma 2
- Bcl-XL/BCL2L1:
-
BCL2-like 1
- Bid:
-
BH3-interacting domain death agonist
- DcR:
-
decoy receptor
- DISC:
-
death-inducing signaling complex
- DR:
-
death receptor
- FADD:
-
Fas-associated DD kinase
- FLICE:
-
FADD-like interleukin-1 α-converting enzyme
- c-FLIP:
-
cellular FLICE-like inhibitory protein
- IC50:
-
concentration giving 50% growth inhibition
- Δψm:
-
mitochondrial transmembrane potential
- PARP:
-
poly-ADP-ribose polymerase
- PCA:
-
principal component analysis
- PDGFRs:
-
platelet-derived growth factor receptors
- PI:
-
propidium iodide
- TRAIL:
-
tumor necrosis factor-related apoptosis-inducing ligand
- TRAIL-R:
-
TRAIL receptor
References
Al-Shahrour F, Diaz-Uriarte R, Dopazo J . (2004). Bioinformatics 20: 578–580.
All-Ericsson C, Girnita L, Muller-Brunotte A, Brodin B, Seregard S, Ostman A et al. (2004). Invest Ophthalmol Vis Sci 45: 2075–2082.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA et al. (1999). J Clin Invest 104: 155–162.
Barnhart BC, Lee JC, Alappat EC, Peter ME . (2003). Oncogene 22: 8634–8644.
Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M et al. (2000). Nature 406: 536–540.
Carcelain G, Rouas-Freiss N, Zorn E, Chung-Scott V, Viel S, Faure F et al. (1997). Int J Cancer 72: 241–247.
Carr KM, Bittner M, Trent JM . (2003). Oncogene 205722: 3076–3080.
Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB et al. (1997). Blood 90: 4947–4952.
Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC . (2004). Cell Death Differ 11: 915–923.
Clark EA, Golub TR, Lander ES, Hynes RO . (2000). Nature 406: 532–535.
Cory GO, Ridley AJ . (2002). Nature 418: 732–733.
Debatin KM, Krammer PH . (2004). Oncogene 23: 2950–2966.
Decaudin D, de Cremoux P, Sastre X, Judde JG, Nemati F, Tran-Perennou C et al. (2005). Int J Cancer 113: 849–856.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S et al. (1999). J Cell Biol 144: 891–901.
Diarra-Mehrpour M, Arrabal S, Jalil A, Pinson X, Gaudin C, Pietu G et al. (2004). Cancer Res 64: 719–727.
Druker BJ . (2002). Oncogene 21: 8541–8546.
Dufour E, Carcelain G, Gaudin C, Flament C, Avril MF, Faure F . (1997). J Immunol 158: 3787–3795.
El-Deiry WS . (2001). Cell Death Differ 8: 1066–1075.
Eriksson L, Antti H, Gottfries J, Holmes E, Johansson E, Lindgren F et al. (2004). Anal Bioanal Chem 380: 419–429.
Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC et al. (2004). Cell Death Differ 11 (Suppl 1): S86–S96.
Gross A, McDonnell JM, Korsmeyer SJ . (1999). Genes Dev 13: 1899–1911.
Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H et al. (1996). Cancer Res 56: 1713–1718.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ . (2000). Blood 96: 925–932.
Hersey P, Zhang XD . (2001). Nat Rev Cancer 1: 142–150.
Hodgkinson CA, Nakayama A, Li H, Swenson LB, Opdecamp K, Asher Jr JH et al. (1993). Cell Death Differ 74: 395–404.
Ivanov VN, Bhoumik A, Ronai Z . (2003). Oncogene p205722: 3152–3161.
Ivanov VN, Hei TK . (2005). Oncogene 24: 616–626.
Jin TG, Kurakin A, Benhaga N, Abe K, Mohseni M, Sandra F et al. (2004). J Biol Chem 279: 55594–55601.
Johnston JB, Kabore AF, Strutinsky J, Hu X, Paul JT, Kropp DM et al. (2003). Oncogene 22: 8356–8369.
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J et al. (2001). J Pharmacol Exp Ther 299: 31–38.
Kim KM, Lee YJ . (2005). Oncogene 24: 355–366.
Kroemer G, Zamzami N, Susin SA . (1997). Immunol Today 18: 44–51.
Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S . (2001). J Biol Chem 276: 20633–20640.
Krystal GW, Honsawek S, Litz J, Buchdunger E . (2000). Clin Cancer Res 6: 3319–3326.
LeBlanc HN, Ashkenazi A . (2003). Cell Death Differ 10: 66–75.
Lefevre G, Glotin AL, Calipel A, Mouriaux F, Tran T, Kherrouche Z et al. (2004). J Biol Chem 279: 31769–31779.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ . (2002). Cancer Cell 2: 183–192.
Leverkus M, Neumann M, Mengling T, Rauch CT, Brocker EB, Krammer PH et al. (2000). Cancer Res 60: 553–559.
Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E, Saikumar P . (2003). J Biol Chem 278: 5367–5376.
Muhlethaler-Mottet A, Bourloud KB, Auderset K, Joseph JM, Gross N . (2004). Oncogene 23: 5415–5425.
Nesterov A, Ivashchenko Y, Kraft AS . (2002). Oncogene 21: 1135–1140.
Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS . (2001). J Biol Chem 276: 10767–10774.
Nimmanapalli R, Bhalla K . (2002). Oncogene 21: 8584–8590.
Pandiella A, Carvajal-Vergara X, Tabera S, Mateo G, Gutierrez N, San Miguel JF . (2003). Br J Haematol 123: 858–868.
Poncet D, Larochette N, Pauleau AL, Boya P, Jalil AA, Cartron PF et al. (2004). J Biol Chem 279 (7): 22605–22614.
Sattler M, Salgia R . (2004). Leuk Res 28 (Suppl 1): S11–S20.
Soengas MS, Lowe SW . (2003). Oncogene 22: 3138–3151.
Strasser A . (2005). Nat Rev Immunol 5: 189–200.
Taylor JR, Brownlow N, Domin J, Dibb NJ . (2006). Oncogene 25: 147–151.
Thomas WD, Zhang XD, Franco AV, Nguyen T, Hersey P . (1998). J Immunol 161: 2195–2200.
Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F et al. (1997). Nature 386: 517–521.
Uziel O, Fenig E, Nordenberg J, Beery E, Reshef H, Sandbank J et al. (2005). Br J Cancer 92: 1881–1891.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al. (1999). Nat Med 5: 157–163.
Wang S, El-Deiry WS . (2003). Oncogene 22: 8628–8633.
Weeraratna AT, Becker D, Carr KM, Duray PH, Rosenblatt KP, Yang S et al. (2004). Oncogene 23: 2264–2274.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M et al. (2000). Genes Dev 14: 2060–2071.
Wendt J, von Haefen C, Hemmati P, Belka C, Dorken B, Daniel PT . (2005). Oncogene 24: 4052–4064.
Wittnebel S, Jalil A, Thiery J, DaRocha S, Viey E, Escudier B et al. (2005). Eur Cytokine Netw 16: 123–127.
Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K . (2004). Cancer Sci 95: 777–783.
Yamaguchi K, Uzzo RG, Pimkina J, Makhov P, Golovine K, Crispen P et al. (2005). Oncogene 24: 5868–5877.
Acknowledgements
We thank Philippe Dessen for his continuous help with microarray data analysis, Nazanine Modjtahedi for helpful discussions, Marie-Dominique Galibert for reading the manuscript and Catherine Gaudin and Thomas Robert for technical assistance. This work was supported by grants from INSERM, Association pour la Recherche sur le Cancer (grant 3520 to MD), and Ligue Nationale Contre le Cancer (Val de Marne 2002). Ahmed Hamaï is a recipient of a fellowship from cancéropole Ile de France and Fondation de France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Hamaï, A., Richon, C., Meslin, F. et al. Imatinib enhances human melanoma cell susceptibility to TRAIL-induced cell death: relationship to Bcl-2 family and caspase activation. Oncogene 25, 7618–7634 (2006). https://doi.org/10.1038/sj.onc.1209738
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209738
Keywords
This article is cited by
-
Synthesis and biological evaluation of phenyl-amino-pyrimidine and indole/oxindole conjugates as potential BCR-ABL inhibitors
Medicinal Chemistry Research (2019)
-
Hypoxia and MITF regulate KIT oncogenic properties in melanocytes
Oncogene (2016)
-
Sensitization of Melanoma Cells for Death Ligand TRAIL Is Based on Cell Cycle Arrest, ROS Production, and Activation of Proapoptotic Bcl-2 Proteins
Journal of Investigative Dermatology (2015)
-
STI571 reduces TRAIL-induced apoptosis in colon cancer cells: c-Abl activation by the death receptor leads to stress kinase-dependent cell death
Journal of Biomedical Science (2012)
-
Imatinib Mesylate Improves Liver Regeneration and Attenuates Liver Fibrogenesis in CCL4-Treated Mice
Journal of Gastrointestinal Surgery (2012)