Abstract
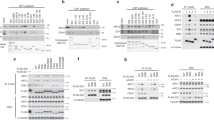
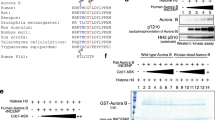
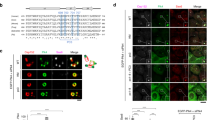
The epithelial cell transforming gene 2 (ECT2) protooncogene encodes a Rho exchange factor, and regulates cytokinesis. ECT2 is phosphorylated in G2/M phases, but its role in the biological function is not known. Here we show that two mitotic kinases, Cdk1 and polo-like kinase 1 (Plk1), phosphorylate ECT2 in vitro. We identified an in vitro Cdk1 phosphorylation site (T412) in ECT2, which comprises a consensus phosphospecific-binding module for the Plk1 polo-box domain (PBD). Endogenous ECT2 in mitotic cells strongly associated with Plk1 PBD, and this binding was inhibited by phosphatase treatment. A phosphorylation-deficient mutant form of ECT2, T412A, did not exhibit strong association with Plk1 PBD compared with wild-type (WT) ECT2. Moreover, ECT2 T412A, but not phosphomimic T412D, displayed a diminished accumulation of GTP-bound RhoA compared with WT ECT2, suggesting that phosphorylation of Thr-412 is critical for the catalytic activity of ECT2. Moreover, while overexpression of WT ECT2 or the T412D mutant caused cortical hyperactivity in U2OS cells during cell division, this activity was not observed in cells expressing ECT2 T412A. These results suggest that ECT2 is regulated by Cdk1 and Plk1 in concert.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bork P, Hofmann K, Bucher P, Neuwald AF, Altschul SF, Koonin EV . (1997). FASEB J 11: 68–76.
Callebaut I, Mornon JP . (1997). FEBS Lett 400: 25–30.
Davis FM, Tsao TY, Fowler SK, Rao PN . (1983). Proc Natl Acad Sci USA 80: 2926–2930.
Elia AE, Cantley LC, Yaffe MB . (2003a). Science 299: 1228–1231.
Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K et al. (2003b). Cell 115: 83–95.
Hall A . (1998). Science 279: 509–514.
Hill CS, Wynne J, Treisman R . (1995). Cell 81: 1159–1170.
Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M et al. (2003). Cell 114: 585–598.
Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S . (2000). J Biol Chem 275: 17233–17236.
Lee JS, Kamijo K, Ohara N, Kitamura T, Miki T . (2004). Exp Cell Res 293: 275–282.
Liu XF, Ishida H, Raziuddin R, Miki T . (2004). Mol Cell Biol 24: 6665–6675.
Maddox AS, Burridge K . (2003). J Cell Biol 160: 255–265.
Miki T, Fleming TP, Bottaro DP, Rubin JS, Ron D, Aaronson SA . (1991). Science 251: 72–75.
Miki T, Smith C, Long J, Eva A, Fleming T . (1993). Nature 362: 462–465.
Miralles F, Posern G, Zaromytidou AI, Treisman R . (2003). Cell 113: 329–342.
Nigg EA . (1998). Curr Opin Cell Biol 10: 776–783.
Nigg EA . (2001). Nat Rev Mol Cell Biol 2: 21–32.
Nigg EA, Blangy A, Lane HA . (1996). Exp Cell Res 229: 174–180.
Reiter NJ, White DJ, Rusnak F . (2002). Biochemistry 41: 1051–1059.
Saito S, Liu XF, Kamijo K, Raziuddin R, Tatsumoto T, Okamoto I et al. (2004). J Biol Chem 279: 7169–7179.
Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T et al. (2002). J Biol Chem 277: 32282–32293.
Seong YS, Min C, Li L, Yang JY, Kim SY, Cao X et al. (2003). Cancer Res 63: 7384–7391.
Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B . (2000). Curr Biol 10: 1162–1171.
Song S, Lee KS . (2001). J Cell Biol 152: 451–469.
Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T . (1999). J Cell Biol 147: 921–927.
Thiel DA, Reeder MK, Pfaff A, Coleman TR, Sells MA, Chernoff J . (2002). Curr Biol 12: 1227–1232.
Wong K, Wessels D, Krob SL, Matveia AR, Lin JL, Soll DR et al. (2000). Cell Motil Cytoskel 45: 121–132.
Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N et al. (2003). J Cell Biol 162: 223–232.
Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K . (2002). Oncogene 21: 8282–8292.
Acknowledgements
We thank Dr Michael Gottesman for support, Dr Keiju Kamijo for assistance with microscopic analysis, and Dr Hiroki Inoue and George Leiman for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Niiya, F., Tatsumoto, T., Lee, K. et al. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene 25, 827–837 (2006). https://doi.org/10.1038/sj.onc.1209124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209124
Keywords
This article is cited by
-
Identification of Metabolic Syndrome-Related miRNA–mRNA Regulatory Networks and Key Genes Based on Bioinformatics Analysis
Biochemical Genetics (2023)
-
Increased expression of ECT2 predicts the poor prognosis of breast cancer patients
Experimental Hematology & Oncology (2022)
-
Mitotic protein kinase-driven crosstalk of machineries for mitosis and metastasis
Experimental & Molecular Medicine (2022)
-
Cytoplasmic expression of epithelial cell transforming sequence 2 in lung adenocarcinoma and its implications for malignant progression
Laboratory Investigation (2019)
-
A cdk1 gradient guides surface contraction waves in oocytes
Nature Communications (2017)