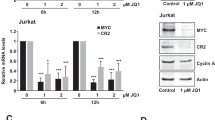
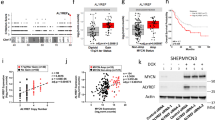
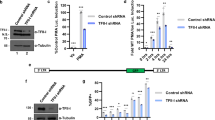
Abstract
The v-Erb A oncoprotein of avian erythroblastosis virus is derived from c-Erb A, a hormone-activated transcription factor. Notably, v-Erb A has sustained multiple mutations relative to c-Erb A and functions as a constitutive transcriptional repressor. We report here an analysis of the contributions of these different mutations to v-Erb A function. Our experiments demonstrate that two amino-acid differences between v-Erb A and c-Erb A, located in the ‘I-box,’ alter the dimerization properties of the viral protein, resulting in more stable homodimer formation, increased corepressor binding, and increased target gene repression. An additional amino-acid difference between v- and c-Erb A, located in helix 3 of the hormone binding domain, renders corepressor binding by the viral protein more resistant to release by thyroid hormone. Finally, we report that a C-terminal truncation in v-Erb A not only inhibits exchange of corepressor and coactivator, as previously noted, but also permits v-Erb A to recruit both SMRT and N-CoR corepressors, whereas c-Erb A is selective for N-CoR. The latter two mutations in v-Erb A also impair its ability to suppress c-Jun function in response to T3 hormone. We propose that the acquisition of oncogenic potential by the v-Erb A protein was a multistep process involving a series of mutations that alter the transcriptional repressive properties of the viral protein through multiple mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Au-Fliegner M, Helmer E, Casanova J, Raaka BM and Samuels HH . (1993). Mol. Cell. Biol., 13, 5725–5737.
Baniahmad A, Kohne AC and Renkawitz R . (1992). EMBO J., 11, 1015–1023.
Bauer A, Mikulits W, Lagger G, Stengl G, Brosch G and Beug H . (1998). EMBO J., 17, 4291–4303.
Bauer A, Ulrich E, Andersson M, Beug H and von Lindern M . (1997). Oncogene, 15, 701–715.
Beug H, Bauer A, Dolznig H, von Lindern M, Lobmayer L, Mellitzer G, Steinlein P, Wessely O and Mullner E . (1996). Biochim. Biophys. Acta, 1288, M35–M47.
Chaterjee VKK . (1997). Hormone Res., 48, 43–46.
Chen H, Smit-McBride Z, Lewis S, Sharif M and Privalsky ML . (1993). Mol. Cell. Biol., 13, 2366–2376.
Chen HW and Privalsky ML . (1993). Mol. Cell. Biol., 13, 5970–5980.
Cohen RN, Brzostek S, Kim B, Chorev M, Wondisford FE and Hollenberg AN . (2001). Mol. Endocrinol., 15, 1049–1061.
Cohen RN, Putney A, Wondisford FE and Hollenberg AN . (2000). Mol. Endocrinol., 14, 900–914.
Cohen RN, Wondisford FE and Hollenberg AN . (1998). Mol. Endocrinol., 12, 1567–1581.
Damm K, Beug H, Graf T and Vennstrom B . (1987). EMBO J., 6, 375–382.
Damm K, Thompson CC and Evans RM . (1989). Nature, 339, 593–597.
Darling DS, Carter RL, Yen PM, Welborn JM, Chin WW and Umeda PK . (1993). J. Biol. Chem., 268, 10221–10227.
DeGroot LJ . (1996). Ann. Intern. Med., 125, 623.
Desbois C, Pain B, Guilhot C, Benchaibi M, Ffrench M, Ghysdael J, Madjar JJ and Samarut J . (1991). Oncogene, 6, 2129–2135.
Disela C, Glineur C, Bugge T, Sap J, Stengl G, Dodgson J, Stunnenberg H, Beug H and Zenke M . (1991). Genes Dev., 5, 2033–2047.
Evans RM . (1989). Int. J. Cancer Suppl., 4, 26–28.
Farboud B and Privalsky ML . (2004). Mol. Endocrinol., 18, 2839–2853.
Forman BM, Casanova J, Raaka BM, Ghysdael J and Samuels HH . (1992). Mol. Endocrinol., 6, 429–442.
Forman BM and Samuels HH . (1990). New Biol., 2, 587–594.
Forman BM, Yang CR, Au M, Casanova J, Ghysdael J and Samuels HH . (1989). Mol. Endocrinol., 3, 1610–1626.
Forrest D, Munoz A, Raynoschek C, Vennstrom B and Beug H . (1990). Oncogene, 5, 309–316.
Gandrillon O, Ferrand N, Michaille JJ, Roze L, Zile MH and Samarut J . (1994). Oncogene, 9, 749–758.
Gandrillon O, Jurdic P, Benchaibi M, Xiao JH, Ghysdael J and Samarut J . (1987). Cell, 49, 687–697.
Gandrillon O, Jurdic P, Pain B, Desbois C, Madjar JJ, Moscovici MG, Moscovici C and Samarut J . (1989). Cell, 58, 115–121.
Ghysdael J and Beug H . (1992). Cancer Surv., 14, 169–180.
Glass CK . (1994). Endocr. Rev., 15, 391–407.
Glass CK . (1996). J. Endocrinol., 150, 349–357.
Glass CK, Lipkin SM, Devary OV and Rosenfeld MG . (1989). Cell, 59, 697–708.
Glass CK and Rosenfeld MG . (2000). Genes Dev., 14, 121–141.
Goodson ML, Jonas BA and Privalsky ML . (2005). J. Biol. Chem., 280, 7493–7503.
Graf T and Beug H . (1983). Cell, 34, 7–9.
Guan KL and Dixon JE . (1991). Anal. Biochem., 192, 262–267.
Harbers M, Wahlstrom GM and Vennstrom B . (1998). J. Steroid Biochem. Mol. Biol., 67, 181–191.
Hauksdottir H and Privalsky ML . (2001). Cell Growth Differ., 12, 85–98.
Judelson C and Privalsky ML . (1996). J. Biol. Chem., 271, 10800–10805.
Katz RW and Koenig RJ . (1994). J. Biol. Chem., 269, 18915–18920.
Keidel S, LeMotte P and Apfel C . (1994). Mol. Cell. Biol., 14, 287–298.
Lazar MA, Berrodin TJ and Harding HP . (1991). Mol. Cell. Biol., 11, 5005–5015.
Lee S and Privalsky ML . (2005). Mol. Endocrinol., 19, 863–878.
Leng X, Tsai SY, O'Malley BW and Tsai MJ . (1993). J. Steroid Biochem. Mol. Biol., 46, 643–661.
Makowski A, Brzostek S, Cohen RN and Hollenberg AN . (2003). Mol. Endocrinol., 17, 273–286.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P and Evans RM . (1995). Cell, 83, 835–839.
Miyamoto T, Suzuki S and DeGroot LJ . (1993). Mol. Endocrinol., 7, 224–231.
Munoz A, Zenke M, Gehring U, Sap J, Beug H and Vennstrom B . (1988). EMBO J., 7, 155–159.
Pain B, Melet F, Jurdic P and Samarut J . (1990). New Biol., 2, 284–294.
Perlmann T, Umesono K, Rangarajan PN, Forman BM and Evans RM . (1996). Mol. Endocrinol., 10, 958–966.
Piedrafita FJ, Bendik I, Ortiz MA and Pfahl M . (1995). Mol. Endocrinol., 9, 563–578.
Pincus MR, Chung D, Dykes DC, Brandt-Rauf P, Weinstein IB, Yamaizumi Z and Nishimura S . (1992). Ann. Clin. Lab. Sci., 22, 323–342.
Privalsky ML . (1992). Biochim. Biophys. Acta, 1114, 51–62.
Privalsky ML . (2004). Annu. Rev. Physiol., 66, 315–360.
Refetoff S . (1993). Clin. Lab. Med., 13, 563–581.
Reginato MJ, Zhang J and Lazar MA . (1996). J. Biol. Chem., 271, 28199–28205.
Ribeiro RC, Apriletti JW, Wagner RL, West BL, Feng W, Huber R, Kushner PJ, Nilsson S, Scanlan T, Fletterick RJ, Schaufele F and Baxter JD . (1998). Recent Prog. Horm. Res., 53, 351–392; discussion 392–354.
Ribeiro RC, Feng W, Wagner RL, Costa CH, Pereira AC, Apriletti JW, Fletterick RJ and Baxter JD . (2001). J. Biol. Chem., 276, 14987–14995.
Ribeiro RC, Kushner PJ, Apriletti JW, West BL and Baxter JD . (1992). Mol. Endocrinol., 6, 1142–1152.
Rietveld LE, Caldenhoven E and Stunnenberg HG . (2001). Oncogene, 20, 3100–3109.
Saatcioglu F, Lopez G, West BL, Zandi E, Feng W, Lu H, Esmaili A, Apriletti JW, Kushner PJ, Baxter JD and Karin M . (1997). Mol. Cell. Biol., 17, 4687–4695.
Samarut J . (1996). Curr. Top. Microbiol. Immunol., 212, 163–173.
Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H and Vennstrom B . (1986). Nature, 324, 635–640.
Sap J, Munoz A, Schmitt J, Stunnenberg H and Vennstrom B . (1989). Nature, 340, 242–244.
Schroeder C, Gibson L and Beug H . (1992a). Oncogene, 7, 203–216.
Schroeder C, Gibson L, Zenke M and Beug H . (1992b). Oncogene, 7, 217–227.
Selmi S and Samuels HH . (1991). J. Biol. Chem., 266, 11589–11593.
Sharif M and Privalsky ML . (1992). Oncogene, 7, 953–960.
Shen Q and Subauste JS . (2000). J. Biol. Chem., 275, 41018–41027.
Soucek L and Evan G . (2002). Cancer Cell, 1, 406–408.
Starr DB, Matsui W, Thomas JR and Yamamoto KR . (1996). Genes Dev., 10, 1271–1283.
Stunnenberg HG, Garcia-Jimenez C and Betz JL . (1999). Biochim. Biophys. Acta, 1423, F15–F33.
Subauste JS and Koenig RJ . (1995). J. Biol. Chem., 270, 7957–7962.
Subauste JS and Koenig RJ . (1998). Mol. Endocrinol., 12, 1380–1392.
Thormeyer D and Baniahmad A . (1999). Int. J. Mol. Med., 4, 351–358.
Tzagarakis-Foster C and Privalsky ML . (1998). J. Biol. Chem., 273, 10926–10932.
Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, Downward J, Mayes ELV, Whittle N, Waterfield D and Seeburg PH . (1984). Nature, 309, 418–425.
Urnov FD, Yee J, Sachs L, Collingwood TN, Bauer A, Beug H, Shi YB and Wolffe AP . (2000). EMBO J., 19, 4074–4090.
Usala SJ . (1991). Thyroid, 1, 361–367.
Vennstrom B, Beug R, Damm K, Engel D, Gehring U, Graf T, Munoz A, Sap J and Zenke M . (1987). Horm. Metab. Res. Suppl., 17, 14–19.
Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD and Fletterick RJ . (1995). Nature, 378, 690–697.
Wahlstrom GM, Harbers M and Vennstrom B . (1996). Oncogene, 13, 843–852.
Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, Baxter JD and Kushner PJ . (2000). Mol. Endocrinol., 14, 1976–1985.
Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ and Evans RM . (1986). Nature, 324, 641–646.
Wondisford FE . (2003). J. Investig. Med., 51, 215–220.
Yen PM, Ikeda M, Brubaker JH, Forgione M, Sugawara A and Chin WW . (1994). J. Biol. Chem., 269, 903–909.
Yen PM, Sugawara A and Chin WW . (1992). J. Biol. Chem., 267, 23248–23252.
Yoh SM and Privalsky ML . (2001). J. Biol. Chem., 276, 16857–16867.
Yoh SM and Privalsky ML . (2002). Methods Mol. Biol., 202, 129–152.
Zamir I, Zhang J and Lazar MA . (1997). Genes Dev., 11, 835–846.
Zenke M, Kahn P, Disela C, Vennstrom B, Leutz A, Keegan K, Hayman MJ, Choi HR, Yew N, Engel JD and Beug H . (1988). Cell, 52, 107–119.
Zenke M, Munoz A, Sap J, Vennstrom B and Beug H . (1990). Cell, 61, 1035–1049.
Zhang J and Lazar MA . (2000). Annu. Rev. Physiol., 62, 439–466.
Zubkova I and Subauste JS . (2002). Biochem. Biophys. Res. Commun., 294, 35–41.
Zubkova I and Subauste JS . (2003). Mol. Cell. Endocrinol., 199, 61–72.
Zubkova I and Subauste JS . (2004). Mol. Biol. Rep., 31, 131–137.
Acknowledgements
We thank Liming Liu for superb technical assistance and KR Yamamoto for the generous gift of the AP-1-luciferase reporter. This work was supported by Public Health Service/National Institutes of Health award R37-CA53394.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Privalsky, M. Multiple mutations contribute to repression by the v-Erb A oncoprotein. Oncogene 24, 6737–6752 (2005). https://doi.org/10.1038/sj.onc.1208826
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208826