Abstract
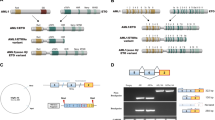
De novo epigenetic changes at histone and DNA level that affect gene transcription in cancer may be less random than we originally thought. Leukemia fusion proteins associated with specific chromosome translocations could mechanistically determine the epigenetic fate of specific target genes critical for normal hematopoiesis. This seems to be the case with AML1-MTG16, a fusion protein resulting from the t(16;21) translocation, a hallmark of therapy-related leukemia and myelodysplastic syndrome. Here we show that AML1-MTG16 blocks both myeloid differentiation and proliferation in the 32D/WT1-mouse myeloid cell line. These biological effects can be traced to the AML1 and MTG16 moieties of the fusion protein, respectively. Further, we show that AML1-MTG16 can induce epigenetic repressive changes at the histone and DNA level of the AML1 target gene Csf1r (c-fms), encoding the macrophage colony stimulating factor receptor. We observed that, concomitant with Csf1r downregulation, 32D/WT1 cells lost the ability to undergo myeloid differentiation in response to the granulocyte macrophage colony-stimulating factor (GM-CSF). Thus, there seems to be an association between AML1-MTG16-induced myeloid maturation block and epigenetic changes of a myeloid master gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ahn MY, Huang G, Bae SC, Wee HJ, Kim WY and Ito Y . (1998). Proc. Natl. Acad. Sci. USA, 95, 1812–1817.
Baylin S and Bestor TH . (2002). Cancer Cell, 1, 299–305.
Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, Su Huang H, Petrelli NJ, Zhang X, O’Dorisio MS, Held WA, Cavenee WK and Plass C . (2000). Nat. Genet., 24, 132–138.
de Koning JP, Soede-Bobok AA, Schelen AM, Smith L, van Leeuwen D, Santini V, Burgering BM, Bos JL, Lowenberg B and Touw IP . (1998). Blood, 91, 1924–1933.
Di Croce L, Raker VA, Corsaro M, Fazi F, Fanelli M, Faretta M, Fuks F, Lo Coco F, Kouzarides T, Nervi C, Minucci S and Pelicci PG . (2002). Science, 295, 1079–1082.
Erickson P, Gao J, Chang KS, Look T, Whisenant E, Raimondi S, Lasher R, Trujillo J, Rowley J and Drabkin H . (1992). Blood, 80, 1825–1831.
Fazi F, Travaglini L, Carotti D, Palitti F, Diverio D, Alcalay M, McNamara S, Miller WH, Coco FL, Pelicci PG and Nervi C . (2005). Oncogene, 24, 1820–1830.
Felgner J, Kreipe H, Heidorn K, Jaquet K, Zschunke F, Radzun HJ and Parwaresch MR . (1991). Pathobiology, 59, 293–298.
Follows GA, Tagoh H, Lefevre P, Hodge D, Morgan GJ and Bonifer C . (2003). EMBO J., 22, 2798–2809.
Gamou T, Kitamura E, Hosoda F, Shimizu K, Shinohara K, Hayashi Y, Nagase T, Yokoyama Y and Ohki M . (1998). Blood, 91, 4028–4037.
Heidenreich O, Krauter J, Riehle H, Hadwiger P, John M, Heil G, Vornlocher HP and Nordheim A . (2003). Blood, 101, 3157–3163.
Herman JG, Graff JR, Myohanen S, Nelkin BD and Baylin SB . (1996). Proc. Natl. Acad. Sci. USA, 93, 9821–9826.
Hoogeveen AT, Rossetti S, Stoyanova V, Schonkeren J, Fenaroli A, Schiaffonati L, Van Unen L and Sacchi N . (2002). Oncogene, 21, 6703–6712.
Jaenisch R and Bird A . (2003). Nat. Genet., 33 (Suppl), 245–254.
Kohzaki H, Ito K, Huang G, Wee HJ, Murakami Y and Ito Y . (1999). Oncogene, 18, 4055–4062.
Kozu T, Miyoshi H, Shimizu K, Maseki N, Kaneko Y, Asou H, Kamada N and Ohki M . (1993). Blood, 82, 1270–1276.
Lutterbach B, Westendorf JJ, Linggi B, Patten A, Moniwa M, Davie JR, Huynh KD, Bardwell VJ, Lavinsky RM, Rosenfeld MG, Glass C, Seto E and Hiebert SW . (1998). Mol. Cell. Biol., 18, 7176–7184.
Meyers S, Downing JR and Hiebert SW . (1993). Mol. Cell. Biol., 13, 6336–6345.
Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y and Ohki M . (1991). Proc. Natl. Acad. Sci. USA, 88, 10431–10434.
Nisson PE, Watkins PC and Sacchi N . (1992). Cancer Genet. Cytogenet., 63, 81–88.
Pabst T, Mueller BU, Harakawa N, Schoch C, Haferlach T, Behre G, Hiddemann W, Zhang DE and Tenen DG . (2001). Nat. Med., 7, 444–451.
Rossetti S, Hoogeveen AT and Sacchi N . (2004). Genomics, 84, 1–9.
Shi H, Wei SH, Leu YW, Rahmatpanah F, Liu JC, Yan PS, Nephew KP and Huang TH . (2003). Cancer Res., 63, 2164–2171.
Tagoh H, Himes R, Clarke D, Leenen PJ, Riggs AD, Hume D and Bonifer C . (2002). Genes Dev., 16, 1721–1737.
Zent CS, Mathieu C, Claxton DF, Zhang DE, Tenen DG, Rowley JD and Nucifora G . (1996). Proc. Natl. Acad. Sci. USA, 93, 1044–1048.
Zhang DE, Fujioka K, Hetherington CJ, Shapiro LH, Chen HM, Look AT and Tenen DG . (1994). Mol. Cell. Biol., 14, 8085–8095.
Zhang DE, Hetherington CJ, Meyers S, Rhoades KL, Larson CJ, Chen HM, Hiebert SW and Tenen DG . (1996). Mol. Cell. Biol., 16, 1231–1240.
Acknowledgements
We are indebted to the Department of Clinical Genetics of the Erasmus Medical Center, Rotterdam, for generous hospitality to SR. We thank Violeta Stoyanova and Gert Jan van de Geijn for critical discussions and technical advice. A special thanks to Ms. Ellen Sanders-Noonan for editing this manuscript. This work was partially supported by an AIRC award to NS and a fellowship from the Center of Excellence CISI, University of Milan to SR.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rossetti, S., Van Unen, L., Touw, I. et al. Myeloid maturation block by AML1-MTG16 is associated with Csf1r epigenetic downregulation. Oncogene 24, 5325–5332 (2005). https://doi.org/10.1038/sj.onc.1208651
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208651
Keywords
This article is cited by
-
UBC9 inhibits myeloid differentiation in collaboration with AML1-MTG8
International Journal of Hematology (2022)
-
A distinct epigenetic signature at targets of a leukemia protein
BMC Genomics (2007)