Abstract
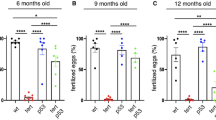
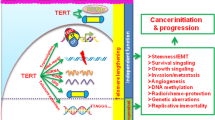
Many degenerative diseases that occur with aging, as well as premature aging syndromes, are characterized by presenting cells with critically short telomeres. Telomerase reintroduction is envisioned as a putative therapy for diseases characterized by telomere exhaustion. K5-mTert transgenic mice overexpress telomerase in a wide spectrum of tissues. These mice have a higher incidence of both induced and spontaneous tumors, resulting in increased mortality during the first year of life. Here, we show that in spite of this elevated tumor incidence and the initial lower survival, K5-mTert mice show an extension of the maximum lifespan from 1.5 to 3 months, depending on the transgenic line, which represents up to a 10% increase in the mean lifespan compared to wild-type littermates. This longer lifespan is coincidental with a lower incidence of certain age-related degenerative diseases, mainly those related to kidney function and germline integrity. Importantly, these effects of telomerase overexpression cannot be attributed to dramatic differences in telomere length in aged K5-Tert mice compared to wild-type mice, as shown by quantitative telomeric FISH. These findings indicate that telomerase overexpression extends the maximum lifespan of mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Artandi SE, Alson S, Tietze MK, Sharpless NE, Ye S, Greenberg RA, Castrillon DH, Horner JW, Weiler SR, Carrasco RD and DePinho RA . (2002). Proc. Natl. Acad. Sci. USA, 99, 8191–8196.
Aviv A and Aviv H . (1998). Hum. Genet., 103, 2–4.
Blackburn EH . (2001). Cell, 106, 661–673.
Blasco MA . (2002). Nat. Rev. Cancer, 2, 627–633.
Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA and Greider CW . (1997). Cell, 91, 25–34.
Canela A, Martín-Caballero J, Flores JM and Blasco M . (2004). Mol. Cell. Biol., 24, 4275–4293.
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A and Kerber RA . (2003). Lancet, 361, 393–395.
Chan SW and Blackburn EH . (2002). Oncogene, 21, 553–563.
Choi D, Whittier PS, Oshima J and Funk WD . (2001). FASEB J., 15, 1014–1020.
Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E and Dym M . (2002). Science, 297, 392–395.
Gonzalez-Suarez E, Flores JM and Blasco MA . (2002). Mol. Cell. Biol., 22, 7291–7301.
Gonzalez-Suarez E, Goytisolo FA, Flores JM and Blasco MA . (2003). Cancer Res., 63, 7047–7050.
Gonzalez-Suarez E, Samper E, Flores JM and Blasco MA . (2000). Nat. Genet., 26, 114–117.
Gonzalez-Suarez E, Samper E, Ramirez A, Flores JM, Martin-Caballero J, Jorcano JL and Blasco MA . (2001). EMBO J., 20, 2619–2630.
Goytisolo FA, Samper E, Martin-Caballero J, Finnon P, Herrera E, Flores JM, Bouffler SD and Blasco MA . (2000). J. Exp. Med., 192, 1625–1636.
Greider CW and Blackburn EH . (1985). Cell, 43, 405–413.
Harley CB, Futcher AB and Greider CW . (1990). Nature, 345, 458–460.
Herrera E, Samper E and Blasco MA . (1999a). EMBO J., 18, 1172–1181.
Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW and Blasco MA . (1999b). EMBO J., 18, 2950–2960.
Lee HW, Blasco MA, Gottlieb GJ, Horner II JW, Greider CW and DePinho RA . (1998). Nature, 392, 569–574.
Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F., Nadal-Ginard B, Kajstura J, Anversa P and Blasco MA . (2003). EMBO J., 22, 131–139.
Lu C, Fu W and Mattson MP . (2001). Brain Res. Dev. Brain Res., 131, 167–171.
Mattson MP and Klapper W . (2001). J. Neurosci. Res., 63, 1–9.
Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP and Ward JM . (1996). Pathobiology of the Aging Mouse Vol 2, ILSI Press, pp. 505.
Moreno E and Basler K . (2004). Cell, 117, 117–129.
O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH, Crispin DA, Potter JD and Rabinovitch PS . (2002). Nat. Genet., 32, 280–284.
Ouellette MM, McDaniel LD, Wright WE, Shay JW and Schultz RA . (2000). Hum. Mol. Genet., 9, 403–411.
Roussel MF . (2003). Cancer Cell, 2, 434–435.
Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C and DePinho RA . (1999). Cell, 96, 701–712.
Ruiz-Torres MP, Perez-Rivero G, Franco S, Blasco MA, Diez-Marques ML and Rodriguez-Puyol D . (2002). J. Am. Soc. Nephrol., 13, 163A.
Samper E, Flores JM and Blasco MA . (2001). EMBO Rep., 2, 800–807.
Samper E, Goytisolo FA, Slijepcevic P, van Buul PP and Blasco MA . (2000). EMBO Rep., 1, 244–252.
Smith LL, Coller HA and Roberts JM . (2003). Nat. Cell Biol., 5, 474–479.
Wyllie FS, Jones CJ, Skinner JW, Haughton MF, Wallis C, Wynford-Thomas D, Faragher RG and Kipling D . (2000). Nat. Genet., 24, 16–17.
Acknowledgements
We are indebted to Rosa Serrano, Elisa Santos, and Jessica Freire for mouse care and genotyping. We thank Todd Devries for statistical calculations and discussion, and Susa Alcamí for proofreading of the manuscript. EG-S is a predoctoral fellow of the Fondo de Investigaciones Sanitarias (FIS). CG is supported by a Marie Curie Fellowship within the 6th EU Framework Programme. MAB laboratory is funded by The Swiss Bridge Cancer Research Award 2000, by The Josef Steiner Cancer Research Award 2003, by grants GEN20014856C1308 and SAF20011869 from Ministry of Science and Technology of Spain, by grant 08.1/0054/01 from the CAM, by EU grants FIGHCT199900009, FIGHCT199900002, QLG1199901341, FIS5200200078, and by the Department of Immunology and Oncology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
González-Suárez, E., Geserick, C., Flores, J. et al. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene 24, 2256–2270 (2005). https://doi.org/10.1038/sj.onc.1208413
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208413
Keywords
This article is cited by
-
Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish
Nature Aging (2023)
-
Mice with hyper-long telomeres show less metabolic aging and longer lifespans
Nature Communications (2019)
-
Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations
Nature Communications (2016)
-
Polymorphisms within the Telomerase Reverse Transcriptase gene (TERT) in four breeds of dogs selected for difference in lifespan and cancer susceptibility
BMC Veterinary Research (2014)
-
Is rate of skin wound healing associated with aging or longevity phenotype?
Biogerontology (2011)