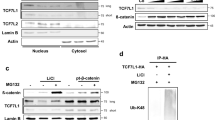
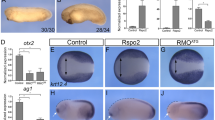
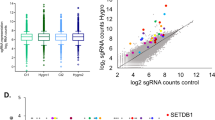
Abstract
Wnt signaling plays an important role in embryonic development and tumorigenesis. These biological effects are exerted by activation of the β-catenin/TCF transcription complex and consequent regulation of a set of downstream genes. TCF-binding elements have been found in the promoter regions of many TCF target genes and characterized by a highly conserved consensus sequence. Utilizing this consensus sequence, we performed an in silico screening for new TCF target genes. Through computational screening and subsequent experimental analysis, we identified a novel TCF target gene, DKK1, which has been shown to be a potent inhibitor of Wnt signaling. Our finding suggests the existence of a novel feedback loop in Wnt signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B and Herrmann BG . (2003). Dev. Cell, 4, 395–406.
Brannon M, Gomperts M, Sumoy L, Moon RT and Kimelman D . (1997). Genes Dev., 11, 2359–2370.
de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A and Perret C . (1998). Proc. Natl. Acad. Sci. USA, 95, 8847–8851.
Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, Kraus MH and Aaronson SA . (1999). J. Biol. Chem., 274, 19465–19472.
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C and Niehrs C . (1998). Nature, 391, 357–362.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW . (1998). Science, 278, 1606–1609.
Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM and Klein PS . (1997). Dev. Biol., 185, 82–91.
Jho EH, Zhang T, Domon C, Joo CK, Freund JN and Costantini F . (2002). Mol. Cell. Biol., 22, 1172–1183.
Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O and Akiyama T . (2000). Science, 289, 1194–1197.
Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D and Pietsch T . (1999). Cancer Res., 59, 269–273.
Loots GG, Locksley RM, Blankespoor CM, Wang ZE, Miller W, Rubin EM and Frazer KA . (2000). Science, 288, 136–140.
Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W and Behrens J . (2002). Mol. Cell. Biol., 22, 1184–1193.
Mao B, Wu W, Davidson G., Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A and Niehrs C . (2002). Nature, 417, 664–667.
Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A and Niehrs C . (2001). Nature, 411, 321–325.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B and Kinzler KW . (1997). Science, 275, 1787–1790.
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC and Westphal H . (2001). Dev. Cell, 1, 423–434.
Niwa H, Burdon T, Chambers I and Smith A . (1998). Genes Dev., 12, 2048–2060.
Paddison PJ, Caudy AA, Bernstein E, Hannon GJ and Conklin DS . (2002). Genes Dev., 16, 948–958.
Pennacchio LA and Rubin EM . (2001). Nat. Rev. Genet., 2, 100–109.
Polakis P . (2000). Genes Dev., 14, 1837–1851.
Sekiya T, Adachi S, Kohu K, Yamada T, Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S, Ohwada S and Akiyama T . (2003). J. Biol. Chem., 279, 6840–6846.
Shinya M, Eschbach C, Clark M, Lehrach H and Furutani-Seiki M . (2000). Mech. Dev., 98, 3–17.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A . (1999). Proc. Natl. Acad. Sci. USA, 96, 5522–5527.
Suzuki Y, Yamashita R, Nakai K and Sugano S . (2002). Nucleic Acids Res., 30, 328–331.
Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H and Akiyama T . (2000). Genes Dev., 14, 1741–1749.
Tetsu O and McCormick F . (1999). Nature, 398, 422–426.
The FANTOM Consortium and the RIKEN Genome Exploration Research Group Phase I & II Team (2002). Nature, 420, 563–573.
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M and Clevers H . (1997). Cell, 88, 789–799.
van de Wetering M, Oosterwegel M, Dooijes D and Clevers H . (1991). EMBO J., 10, 123–132.
Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, Albrecht S, Von Schweinitz D and Pietsch T . (2003). Lab. Invest., 83, 429–434.
Wodarz A and Nusse R . (1998). Annu. Rev. Cell Dev. Biol., 14, 59–88.
Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ and Williams LT . (2001). Proc. Natl. Acad. Sci. USA, 98, 14973–14978.
Acknowledgements
We thank S Adachi, K Satoh, A Nishida and T Moriguchi for helpful discussion. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas and the Organization for Pharmaceutical Safety and Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niida, A., Hiroko, T., Kasai, M. et al. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 23, 8520–8526 (2004). https://doi.org/10.1038/sj.onc.1207892
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207892