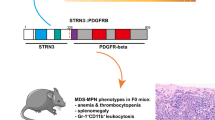
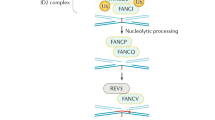
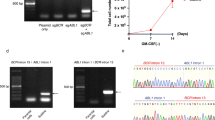
Abstract
A region of 150 kb has been analysed around a previously isolated, lymphoma associated, translocation breakpoint located at chromosome band 11q23. This balanced and reciprocal translocation, t(11;14)(q32;q23), has been shown to result in the fusion between chromosome 11 specific sequence and the switch γ4 region of the IGH locus. The LPC gene, encoding a novel proprotein convertase belonging to the furin family, has been identified in this region. In order to characterize further the region surrounding the translocation, we have determined the detailed structure of LPC. Here we show that LPC consists of at least 16 exons covering 25 kb, and that there is a partial duplication, involving mobile genetic elements and containing LPC exons 13 – 17 in a tail – tail configuration at 65 kb downstream. Since the chromosomal breakpoint lay between these two structures, the intervening region was further analysed and shown to contain at least two unrelated genes. The previously known SM22 gene was localized close to the 3′ tail of LPC. Furthermore, we identified the gene encoding the α2 subunit of platelet-activating factor acetylhydrolase (Pafah1a2) at the chromosomal breakpoint. The position of another previously identified breakpoint was also located to within the first intron of this gene. Altogether, our results give evidence of a genomic instability of this area of 11q23 and show that Pafah1a2 and not LPC is the gene disrupted by the translocation, suggesting that deregulated Pafah1a2 may have a role in lymphomagenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adachi H, Tsujimoto M, Hattori M, Arai H and Inoue K. . 1997 Biochem. Biophys. Res. Com. 233: 10–13.
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD and Brinster RL. . 1985 Nature 318: 533–538.
Akao Y, Tsujimoto Y, Seto M, Imai T, Bergeron D, Berbeau B and Otsuki Y. . 1993 Genes Chrom. Cancer 8: 167–171.
Baron BW, Nucifora G, McCabe N, Espinosa RD, Le Beau MM and McKeithan TW. . 1993 Proc. Natl. Acad. Sci. USA 90: 5262–5266.
Bazan NG. . 1995 Nature 374: 501–502.
Bennett SA and Birnboim HC. . 1997 Mol. Carcinog. 20: 366–375.
Bennett SAJ, Leite LCC and Birnboim HC. . 1993 Carcinogenesis 14: 1289–1296.
Bernard OA and Berger R . (1995) Genes Chrom. Cancer 13: 75–85.
Blank ML, Cress EA, Whittle T and Snyder F. . 1981 Life Sci. 29: 769–775.
Boeke JD. . 1997 Nature Genet. 16: 6–7.
Brown MA, Xu CF, Nicolai H, Griffiths B, Chambers JA, Black D and Solomon E. . 1996 Oncogene 12: 2507–2513.
Camoretti-Mercado B, Forsythe SM, LeBeau MM, Espinosa RR, Vieira JE, Halayko AJ, Willadsen S, Kurtz B, Ober C, Evans GA, Thweatt R, Shapiro S, Niu Q, Qin Y, Padrid PA and Solway J. . 1998 Genomics 49: 452–457.
Chong SS, Pack SD, Roschke AV, Tanigami A, Carrozzo R, Smith AC, Dobyns WB and Ledbetter DH. . 1997 Human Mol. Genet. 6: 147–155.
Consortium TEPKD. . 1994 Cell 77: 881–894.
Crossen PE, Kennedy MA, Heaton DC and Morrison MJ. . 1993 Genes Chrom. Cancer 8: 60–62.
Denizot Y, Trimoreau F, Duouis F, Verger C and Praloran V. . 1995a Blood 85: 2992–2993.
Denizot Y, Trimoreau F, Duouis F, Verger C and Praloran V. . 1995b Leukemia 9: 1982–1983.
Feng Q, Moran JV, Kazazian Jr HH and Boeke JD. . 1996 Cell 87: 905–916.
Hallenberger S, Moulard M, Sordel M, Klenk HD and Garten W. . 1997 J. Virol. 71: 1036–1045.
Hattori M, Adachi H, Aoki J, Tsujimoto M, Arai H and Inoue K. . 1995 J. Biol. Chem. 270: 31345–31352.
Hattori M, Adachi H, Tsujimoto M, Arai H and Inoue K. . 1994 Nature 370: 216–218.
Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ and Wynshaw-Boris A. . 1998 Nature Genet. 19: 333–339.
Ho YS, Swenson L, Derewenda U, Serre L, Wei Y, Dauter Z, Hattori M, Adachi T, Aoki J, Arai H, Inoue K and Derewenda ZS. . 1997 Nature 385: 89–93.
Jurka J. . 1997 Proc. Natl. Acad. Sci. USA 94: 1872–1877.
Kazazian Jr HH and Moran JV. . 1998 Nature Genet. 19: 19–24.
Kazazian Jr HH, Wong C, Youssoufian H, Scott AF, Phillips DG and Antonarakis SE. . 1988 Nature 332: 164–166.
Kurahashi H, Sakamoto M, Ono J, Honda A, Okada S and Nakamura Y. . 1998 Hum. Genet. 103: 189–192.
Lehrach H. . (1990) In: Genome analysis volume1. Davies KE and Tilghman SM (eds).. Cold Spring Harbor Laboratory Press: Cold Spring Harbor pp.39–81.
Li L, Miano JM, Cserjesi P and Olson EN. . 1996a Circ. Res. 78: 188–195.
Li WH, Ellsworth DL, Krushkal J, Chang BHJ and Hewett-Emmett D. . 1996b Mol. Phylogenet. Evol. 5: 182–187.
Maeda N and Smithies O. . 1986 Ann. Rev. Genet. 20: 81–108.
Meerabux J, Yaspo ML, Roebroek AJ, Van de Ven WJ, Lister TA and Young BD. . 1996 Cancer Res. 56: 448–451.
Meerabux JM, Cotter FE, Kearney L, Nizetic D, Dhut S, Gibbons B, Lister TA and Young BD. . 1994 Oncogene 9: 893–898.
Miki Y, Nishisho I, Horii A, Miyoshi Y, Utsunomiya J, Kinzler KW, Vogelstein B and Nakamura Y. . 1992 Cancer Res. 52: 643–645.
Mitelman F, Mertens F and Johansson B. . 1997 Nature Genet. 15: 417–474.
Moro F, Arrigo G, Fogli A, Bernard L and Carrozzo R. . 1998 Genomics 51: 157–159.
Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT and Ledbetter DH. . 1993 Nature 364: 717–721.
Roth M, Nauck M, Yousefi S, Tamm M, Blaser K, Perruchoud AP and Simon HU. . 1996 J. Exp. Med. 184: 191–201.
Sambrook J, Fritsch EF and Maniatis T. . 1989 Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
Sapir T, Elbaum M and Reiner O. . 1997 EMBO J. 16: 6977–6984.
Sassaman DM, Dombroski BA, Moran JV, Kimberland ML, Naas TP, DeBerardinis RJ, Gabriel A, Swergold GD and Kazazian Jr HH. . 1997 Nature Genet. 16: 37–43.
Schichman SA, Caligiuri MA, Strout MP, Carter SL, Gu Y, Canaani E, Bloomfield CD and Croce CM. . 1994 Cancer Res. 54: 4277–4280.
Seidah NG, Chretien M and Day R. . 1994 Biochimie 76: 197–209.
Shimada A, Ota Y, Sugiyama Y, Sato S, Kume K, Shimizu T and Inoue S. . 1998 J. Invest. Dermatol. 110: 889–893.
Strout MP, Marcucci G, Bloomfield CD and Caligiuri MA. . 1998 Proc. Natl. Acad. Sci. USA 95: 2390–2395.
Toriello HV, Glover TW, Takahara K, Byers PH, Miller DE, Higgins JV and Greenspan DS. . 1996 Nature Genet. 13: 361–365.
Von Lindern M, Breems D, van Baal S, Adriaansen H and Grosveld G. . 1992 Genes Chrom. Cancer 5: 227–234.
Ye BH, Chaganti S, Chang CC, Niu H, Corradini P, Chaganti RS and Dalla-Favera R. . 1995 EMBO J. 14: 6209–6217.
Young BD. . 1992 Bailliere's Clin. Haematol. 5: 881–895.
Acknowledgements
This work is dedicated to Alain Lecointe, Botanist (1943 – 1998). We thank Christine Courtes and Emmanuel Douzery for assistance in the sequencing, and Debra Lillington and Michael Neat for the FISH analysis. We are grateful to Armelle Degeorges, Emmanuel Douzery and Ian Robbins for helpful discussions. NL was supported by a fellowship from the Association de la Recherche contre le Cancer. AH was supported by a grant from the Commission of European Communities, contract number F14P-CT-95-008. This work was supported in part by the Concerted Action, contract number CA-11.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lecointe, N., Meerabux, J., Ebihara, M. et al. Molecular analysis of an unstable genomic region at chromosome band 11q23 reveals a disruption of the gene encoding the α2 subunit of platelet-activating factor acetylhydrolase (Pafah1a2) in human lymphoma. Oncogene 18, 2852–2859 (1999). https://doi.org/10.1038/sj.onc.1202645
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202645
Keywords
This article is cited by
-
Differential expression profiling of head and neck squamous cell carcinoma (HNSCC)
British Journal of Cancer (2003)