Abstract
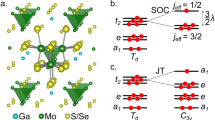
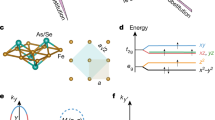
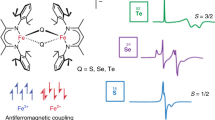
I HAVE followed with considerable interest the discussion which has been recently carried on in NATURE by Messrs. Jackson, Welo and Baudisch on the magnetic properties of the complex compounds of iron and other elements belonging to the first transition group. Two papers on this subject have recently been contributed by me, the first of which was sent in June to the Philosophical Magazine and deals with those complex compounds, the magnetic properties of which have been studied by Rosenbohm and Oxley. I have defined with Sidgwick the “effective atomic number Z′” of the co-ordinating atom in any complex compound, as the sum total of all the electrons contained in the former plus the number of electronic orbits which this atom shares with the neighbouring atoms and groups in the co-ordination compound. It can be shown that Z′ = N E + 2P, where N = atomic number of the co-ordinating atom, E = its primary valency in the given compound and P = 4, 6, etc., according as the complex compound is either fourfold, sixfold, etc.; e.g. in the ferrous compound K4[Fe(CN)6] + 3H2O, Z′ = 26 2 + 2 × 6 = 36; while in the case of the fourfold compound of copper [Cu (NH3)4] (NO3)2, Z′ = 29 2 + 2 × 4 = 35. These numbers can be independently calculated by the rule which has been given by Sidgwick.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BOSE, D. Valence Theories and the Magnetic Properties of Complex Salts. Nature 117, 84 (1926). https://doi.org/10.1038/117084a0
Published:
Issue Date:
DOI: https://doi.org/10.1038/117084a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.