Abstract
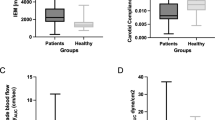
Patients with coronary artery disease (CAD) have impaired endothelial function. Arterial elasticity is modulated by endothelial function. The association between arterial elasticity and endothelial function has not been reported in patients with CAD. The present study was designed to investigate whether endothelial dysfunction contributes to impaired arterial elasticity. Thirty patients with CAD and 30 control subjects were recruited. Large and small artery elasticity indices were non-invasively assessed using pulse wave analysis. Brachial artery endothelium-dependent and -independent function were assessed by vascular response to flow-mediated vasodilation (FMD) and sublingual nitroglyceride (NTG), respectively. C1 large artery elasticity index was not different in the CAD group compared with the control group. However, C2 small artery elasticity index was significantly reduced in the CAD group compared with the control group. Flow-mediated vasodilation (FMD) was also impaired in the CAD group compared with the control group. Flow-mediated vasodilation (FMD) in the brachial artery correlated with C2 small arterial elasticity index. But NTG-mediated brachial artery vasodilation was similar between the two groups. The present findings suggest that the patients with CAD have reduced C2 small arterial elasticity index and impaired FMD. Endothelial dysfunction is involved in diminished arterial elasticity, suggesting that C2 small arterial elasticity index is a novel surrogate measure for the clinical evaluation of endothelial function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davignon J, Ganz P . Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109 (Suppl III): III27–III32.
Behrendt D, Ganz P . Endothelial function. from vascular biology to clinical application. Am J Cardiol 2002; 90: 40L–48L.
Lüscher TF, Barton M . Biology of the endothelium. Clin Cardiol 1997; 20 (Suppl II): II3–II10.
Kinlay S, Libby P, Ganz P . Endothelial function and coronary artery disease. Curr Opin Lipidol 2001; 12: 383–389.
Verma S, Buchanan MR, Anderson TJ . Endothelial function testing as a biomarker of vascular disease. Circulation 2003; 108: 2054–2059.
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A . Long-term follow-up of patients with mild coronary artery disease and endothelial function. Circulation 2000; 101: 948–954.
Schachinger V, Britten MB, Zeiher AM . Prognostic impact of coronary vasodilator dysfunction on adverse longer-term outcome of coronary heart disease. Circulation 2000; 101: 1899–1906.
Fichtlscherer S, Breuer S, Zeiher AM . Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes. further evidence for the existence of the ‘vulnerable’ patients. Circulation 2004; 110: 1926–1932.
Celermajer DS, Sorense KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111–1115.
Raitakari OT, Celermajer DS . Flow-mediated dilatation. Br J Clin Pharmacol 2000; 50: 397–404.
Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995; 26: 1235–1241.
Anderson TJ, Elstein E, Haber H, Charbonneau F . Comparative study of ACE-inhibitor, angiotensin iiantagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease(BANFF study). J Am Coll Cardio 2000; 35: 60–66.
Oliver JJ, Webb DJ . Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vascular Biol 2003; 23: 168–175.
McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis; aging and arterial compliance. Hypertension 1999; 33: 1392–1398.
Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR . Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002; 105: 213–217.
Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 2001; 38: 1049–1053.
Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui N et al. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age. Am J Hypertens 2004; 17: 654–659.
Mc Veigh GE, Allen PB, Morgan DR, Hanratty CG . Silke: nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci 2001; 100: 387–393.
Celermajer DS, Sorense KE, Bull C, Robinson J, Deanfield JE . Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994; 24: 1468–1474.
Avolio AP, Chen S-G, Wang R-P, Zhang CL, Li MF, O'Rouke MF . Effects of ageing on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 1983; 68: 50–58.
Avolin A, Jones D, Tafazzoli-Shadpour M . Quantification of alterations in structure and function of elastin in the arterial media. Hypertension 1998; 32: 170–175.
Syeda B, Gottsauner WM, Denk S, Pichler P, Khorsand A, Glogar D . Arterial compliance: a diagnostic marker for atherosclerotic plaque burden? Am J Hypertens 2003; 16: 356–362.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, J., Liu, DH., Wang, LC. et al. Arterial elasticity identified by pulse wave analysis and its relation to endothelial function in patients with coronary artery disease. J Hum Hypertens 21, 149–153 (2007). https://doi.org/10.1038/sj.jhh.1002112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1002112
Keywords
This article is cited by
-
Increased plasma neopterin levels are associated with reduced endothelial function and arterial elasticity in hypertension
Journal of Human Hypertension (2016)
-
Elevated circulating endothelial microparticles and brachial–ankle pulse wave velocity in well-controlled hypertensive patients
Journal of Human Hypertension (2009)
-
Berberine-induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity
Journal of Human Hypertension (2008)
-
Elasticity Indices of Large and Small Arteries in Relation to the Metabolic Syndrome in Chinese
American Journal of Hypertension (2008)