Abstract
Objective:
The objective of this study was to determine the contribution of mesothelial cells, present in human omental adipose tissue (OAT) but not in the subcutaneous depot (SAT), on the expression of inflammation-related factors.
Design:
Comparison of the expression profiles of inflammation-related genes in mesothelial cells with those in the adipocyte-enriched (AEF) and stromal vascular fractions (SVF) and localization of interleukin-18 (IL-18) expression in adipose depots.
Subjects:
Eleven obese Caucasian female subjects undergoing gastric bypass surgery (body mass index: 43.6±1.3 kg/m2; age: 41.6±2.3 years).
Measurements:
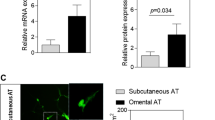
The expression profiles of cytokine and chemokine-related genes in mesothelial cells and in cell fractions prepared from OAT were assessed by the microarray technique. The differential expression of IL-18 was confirmed by real-time PCR and the protein was localized in adipose depots by immunohistochemistry.
Results:
Microarray data analysis demonstrated that, of the 16 cytokine and chemokine-related genes that were upregulated in mesothelial cells compared with the AEF, IL-18 was the cytokine with the highest differential expression. IL-18 expression was similar in mesothelial cells and the SVF. In both SAT and OAT, IL-18 was immunolocalized in neutrophils and mast cells, but not in macrophages nor adipocytes. This cytokine was also detected in mesothelial cells in OAT. This additional source of expression may explain the higher IL-18 expression levels in OAT than SAT (+5.9-fold).
Conclusion:
By their capacity to express inflammatory-related factors, and in particular the proinflammatory cytokine IL-18 in OAT, mesothelial cells appear as a new player in the process of low-grade inflammation associated with obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Trayhurn P, Wood IS . Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355.
Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 2005; 54: 2932–2938.
Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G et al. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab 2002; 87: 3864–3866.
Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL . Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab 2004; 89: 806–811.
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 2006; 12: 650–656.
Skurk T, Herder C, Kraft I, Muller-Scholze S, Hauner H, Kolb H . Production and release of macrophage migration inhibitory factor from human adipocytes. Endocrinology 2005; 146: 1006–1011.
Skurk T, Kolb H, Muller-Scholze S, Rohrig K, Hauner H, Herder C . The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol 2005; 152: 863–868.
Wood IS, Wang B, Jenkins JR, Trayhurn P . The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun 2005; 337: 422–429.
Fain JN, Tichansky DS, Madan AK . Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metabolism 2006; 55: 1113–1121.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Bruun JM, Lihn AS, Pedersen SB, Richelsen B . Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 2005; 90: 2282–2289.
Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006; 55: 1554–1561.
Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res 2004; 12: 1217–1222.
Wilkosz S, Ireland G, Khwaja N, Walker M, Butt R, de Giorgio-Miller A et al. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl) 2005; 209: 251–261.
Williams SK, Wang TF, Castrillo R, Jarrell BE . Liposuction-derived human fat used for vascular graft sodding contains endothelial cells and not mesothelial cells as the major cell type. J Vasc Surg 1994; 19: 916–923.
van Hinsbergh VW, Kooistra T, Scheffer MA, Hajo van Bockel J, van Muijen GN . Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood 1990; 75: 1490–1497.
Mutsaers SE . The mesothelial cell. Int J Biochem Cell Biol 2004; 36: 9–16.
Visser CE, Tekstra J, Brouwer-Steenbergen JJ, Tuk CW, Boorsma DM, Sampat-Sardjoepersad SC et al. Chemokines produced by mesothelial cells: huGRO-alpha, IP-10, MCP-1 and RANTES. Clin Exp Immunol 1998; 112: 270–275.
Chung-Welch N, Patton WF, Shepro D, Cambria RP . Two-stage isolation procedure for obtaining homogenous populations of microvascular endothelial and mesothelial cells from human omentum. Microvasc Res 1997; 54: 121–134.
Takahashi K, Hata J, Mukai K, Sawasaki Y . Close similarity between cultured human omental mesothelial cells and endothelial cells in cytochemical markers and plasminogen activator production. In vitro Cell Dev Biol 1991; 27A: 542–548.
Chung-Welch N, Patton WF, Shepro D, Cambria RP . Human omental microvascular endothelial and mesothelial cells: characterization of two distinct mesodermally derived epithelial cells. Microvasc Res 1997; 54: 108–120.
Mineau-Hanschke R, Patton WF, Hechtman HB, Shepro D . Immunolocalization of cytokeratin 19 in bovine and human pulmonary microvascular endothelial cells in situ. Comp Biochem Physiol Comp Physiol 1993; 104: 313–319.
Hassan R, Bera T, Pastan I . Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10: 3937–3942.
Mansourian R, Mutch DM, Antille N, Aubert J, Fogel P, Le Goff JM et al. The global error assessment (GEA) model for the selection of differentially expressed genes in microarray data. Bioinformatics 2004; 20: 2726–2737.
Darimont C, Avanti O, Zbinden I, Leone-Vautravers P, Mansourian R, Giusti V et al. Liver X receptor preferentially activates de novo lipogenesis in human preadipocytes. Biochimie 2006; 88: 309–318.
Gabrielsson BG, Olofsson LE, Sjogren A, Jernas M, Elander A, Lonn M et al. Evaluation of reference genes for studies of gene expression in human adipose tissue. Obes Res 2005; 13: 649–652.
Gabrielsson BG, Johansson JM, Jennische E, Jernas M, Itoh Y, Peltonen M et al. Depot-specific expression of fibroblast growth factors in human adipose tissue. Obes Res 2002; 10: 608–616.
Bjorntorp P . The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 1996; 20: 291–302.
Yao V, Platell C, Hall JC . Peritoneal mesothelial cells produce inflammatory related cytokines. ANZ J Surg 2004; 74: 997–1002.
Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S . Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol 1999; 162: 7322–7329.
Hutson JC, Stocco DM . Comparison of cellular and secreted proteins of macrophages from the testis and peritoneum on two-dimensional polyacrylamide gels: evidence of tissue specific function. Reg Immunol 1989; 2: 249–253.
Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K . Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol 1998; 70: 281–312.
Lofgren P, van Harmelen V, Reynisdottir S, Naslund E, Ryden M, Rossner S et al. Secretion of tumor necrosis factor-alpha shows a strong relationship to insulin-stimulated glucose transport in human adipose tissue. Diabetes 2000; 49: 688–692.
Rotter V, Nagaev I, Smith U . Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003; 278: 45777–45784.
Acknowledgements
We are grateful to Dr DM Mutch (INSERM U755, Hôpital Hôtel Dieu, Paris, France), Dr K Acheson and F Raymond (Nestlé Research Center, Lausanne, Switzerland) for fruitful discussions and critically reading the manuscript, and C Verdumo (University Hospital CHUV, Lausanne, Switzerland) for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darimont, C., Avanti, O., Blancher, F. et al. Contribution of mesothelial cells in the expression of inflammatory-related factors in omental adipose tissue of obese subjects. Int J Obes 32, 112–120 (2008). https://doi.org/10.1038/sj.ijo.0803688
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803688