Abstract
Objective:
To assess weight maintenance after weight loss by consumption of yoghurt with a novel fat emulsion (Olibra) including effects on body composition, resting energy expenditure (REE), fat oxidation, hunger feelings and satiety hormones.
Design:
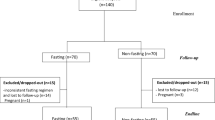
A randomized, placebo-controlled, double-blind, parallel design. A 6-week weight loss period (2.1 MJ/day) was followed by 18 weeks weight maintenance with test (Olibra) or placebo yoghurt.
Subjects:
Fifty overweight women (age: 18–58 years, body mass index (BMI) 25–32 kg/m2).
Measurements:
In weeks 1, 7 and 25, a satiety test with questionnaires and blood samples for analysis of satiety hormones. In weeks 2, 8 and 26, REE, body weight and body composition.
Results:
During weight maintenance after significant body weight reduction, there was no significant increase in body weight in the test group (1.1±3.4 kg); the placebo group did gain weight (3.0±3.1 kg, P<0.001). Compared to the placebo group, the test group was less hungry 4 h after yoghurt consumption in week 25 (P<0.05) and showed increased glucagon like peptide-1 values 180 min after yoghurt consumption (week 25 vs week 1, P<0.05). Measured REE as a function of fat-free mass (FFM) was significantly higher than predicted REE (P<0.05) in week 26 for the test group, but not for the placebo group. Fat mass (FM) was significantly more decreased in the test group (6.5±4.1 kg) compared to the placebo group (4.1±3.6 kg) (week 26 vs week 2, P<0.05).
Conclusion:
Consumption of Olibra yoghurt improved weight maintenance compared to placebo, which can be explained by the relatively higher REE as a function of FFM, relatively higher decrease in FM and the relatively lower increase in hunger.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Seidell JC . Dietary fat and obesity: an epidemiologic perspective. Am J Clin Nutr 1998; 67: 546S–550S.
Stunkard AJ . Current views on obesity. Am J Med 1996; 100: 230–236.
Goldstein DJ . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 1992; 16: 397–415.
Van Gaal LF, Wauters MA, De Leeuw IH . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord 1997; 21 (Suppl 1): S5–S9.
Burns AA, Livingstone MB, Welch RW, Dunne A, Reid CA, Rowland IR . The effects of yoghurt containing a novel fat emulsion on energy and macronutrient intakes in non-overweight, overweight and obese subjects. Int J Obes Relat Metab Disord 2001; 25: 1487–1496.
Burns AA, Livingstone MB, Welch RW, Dunne A, Robson PJ, Lindmark L et al. Short-term effects of yoghurt containing a novel fat emulsion on energy and macronutrient intakes in non-obese subjects. Int J Obes Relat Metab Disord 2000; 24: 1419–1425.
Burns AA, Livingstone MB, Welch RW, Dunne A, Rowland IR . Dose–response effects of a novel fat emulsion (Olibra) on energy and macronutrient intakes up to 36 h post-consumption. Eur J Clin Nutr 2002; 56: 368–377.
Harris JA, Benedict FG . A Biometric Study of Basal Metabolism in Man. Carnegia Institution: Washington, 1919.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Raben A, Holst JJ, Christensen NJ, Astrup A . Determinants of postprandial appetite sensations: macronutrient intake and glucose metabolism. Int J Obes Relat Metab Disord 1995; 20: 161–169.
Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD . Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980; 33: 2686–2693.
van Marken Lichtenbelt WD, Westerterp KR, Wouters L . Deuterium dilution as a method for determining total body water: effect of test protocol and sampling time. Br J Nutr 1994; 72: 491–497.
Westerterp KR, Wouters L, van Marken Lichtenbelt WD . The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water. Obes Res 1995; 3 (Suppl 1): 49–57.
Forsum E, Kabir N, Sadurskis A, Westerterp K . Total energy expenditure of healthy Swedish women during pregnancy and lactation. Am J Clin Nutr 1992; 56: 334–342.
Adriaens MP, Schoffelen PF, Westerterp KR . Intra-individual variation of basal metabolic rate and the influence of daily habitual physical activity before testing. Br J Nutr 2003; 90: 419–423.
Weir JBDV . New methods for calculating metabolic rate with special references to protein metabolism. J Physiol 1949; 109: 1–9.
Ravussin E, Bogardus C . A brief overview of human energy metabolism and its relationship to essential obesity. Am J Clin Nutr 1992; 55: 242S–245S.
Dulloo AG, Jacquet J . The control of partitioning between protein and fat during human starvation: its internal determinants and biological significance. Br J Nutr 1999; 82: 339–356.
Kempen KP, Saris WH, Westerterp KR . Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women. Am J Clin Nutr 1995; 62: 722–729.
Adam TC, Westerterp-Plantenga MS . Nutrient-stimulated GLP-1 release in normal-weight men and women. Horm Metab Res 2005; 37: 111–117.
Lejeune MP, Kovacs EM, Westerterp-Plantenga MS . Additional protein intake limits weight regain after weight loss in humans. Br J Nutr 2005; 93: 281–289.
Westerterp-Plantenga MS, Lejeune MP, Kovacs EM . Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 2005; 13: 1195–1204.
Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM . High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 2004; 28: 57–64.
Visscher TL, Seidell JC . Time trends (1993–1997) and seasonal variation in body mass index and waist circumference in the Netherlands. Int J Obes Relat Metab Disord 2004; 28: 1309–1316.
Bigaard J, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI . Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res 2003; 11: 895–903.
Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL et al. Waist circumference and body composition in relation to all-cause mortality in middle-aged men and women. Int J Obes (Lond) 2005; 29: 778–784.
Logan CM, McCaffrey TA, Wallace JM, Robson PJ, Welch RW, Dunne A et al. Investigation of the medium-term effects of Olibratrade mark fat emulsion on food intake in non-obese subjects. Eur J Clin Nutr 2006; 60: 1081–1091.
Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC et al. The ileal brake – inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984; 25: 365–374.
Van Citters GW, Lin HC . The ileal brake: a fifteen-year progress report. Curr Gastroenterol Rep 1999; 1: 404–409.
Symerski T, Kee B, Haddeman E, Peters H, Masclee A . Ileal brake effects on satiety and meal intake in humans after a meal replacer (abstract). Int J Obes Relat Metab Disord 2004; 28 (Suppl 1): S148.
Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL . Regional distribution and release of peptide YY with fatty acids of different chain length. Am J Physiol 1985; 249: G745–G750.
Jin H, Cai L, Lee K, Chang TM, Li P, Wagner D et al. A physiological role of peptide YY on exocrine pancreatic secretion in rats. Gastroenterology 1993; 105: 208–215.
Acknowledgements
We gratefully acknowledge Roy Langeveld, Manuela Lejeune, Natalie Luscombe-Marsh, Joan Senden, Wendy Sluijsmans, Astrid Smeets, Jos Stegen, Loek Wouters and Peter Zuurendonk. The study was funded by Campina Innovation, Wageningen, The Netherlands. The Olibra fat emulsion was provided by Lipid Technologies Provider AB, Karlshamn, Sweden.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure of conflict of interest
The study was sponsored by Campina Innovation, Wageningen, The Netherlands, which raises potential duality of interest.
Rights and permissions
About this article
Cite this article
Diepvens, K., Soenen, S., Steijns, J. et al. Long-term effects of consumption of a novel fat emulsion in relation to body-weight management. Int J Obes 31, 942–949 (2007). https://doi.org/10.1038/sj.ijo.0803532
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803532
Keywords
This article is cited by
-
Effects of lipid emulsion particle size on satiety and energy intake: a randomised cross-over trial
European Journal of Clinical Nutrition (2018)
-
Timeline of changes in appetite during weight loss with a ketogenic diet
International Journal of Obesity (2017)
-
Effect of a vegetable-oil emulsion on body composition; a 12-week study in overweight women on a meal replacement therapy after an initial weight loss: a randomized controlled trial
European Journal of Nutrition (2011)
-
Making claims: functional foods for managing appetite and weight
Nature Reviews Endocrinology (2010)
-
No effect of an oleoylethanolamide-related phospholipid on satiety and energy intake: a randomised controlled trial of phosphatidylethanolamine
Lipids in Health and Disease (2008)