Abstract
Objective:
The incidence of obesity and overweight in the US has increased considerably during the past two decades and currently affects 65% of the adult population. Research has indicated that small, yet irreversible, gains during the holiday season contribute to increases in weight during adulthood. Conjugated linoleic acid (CLA), a naturally occurring dietary fatty acid, has been found to reduce weight gain and dramatically decrease fat mass in animals. Although research in humans has shown inconsistent results, most studies have been of insufficient duration or have utilized body composition methods that are less accurate than the currently accepted criterion.
Design:
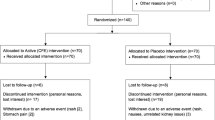
Randomized, double-blind, placebo-controlled study of 3.2 g/day CLA for 6 months.
Subjects:
Forty healthy, overweight subjects (age: 18–44 years; body mass index: 25–30 kg/m2)
Measurements:
Body composition by the four-compartment model, resting metabolic rate (RMR) by indirect calorimetry, self-reported physical activity and dietary intake, and blood chemistries were determined at baseline and after 6 months. Body weight was measured monthly during the pre-holiday season (August–October), holiday season (November–December) and post-holiday season (January–March). Adverse events were assessed monthly.
Results:
Compared to CLA, the placebo group showed a greater rate of weight gain during the holiday season (P=0.01). Within the placebo group, holiday weight change was significantly greater compared to the pre-holiday period (August–October) (P=0.03). Six-month change in body composition was improved with CLA compared to placebo (P=0.02), and body fat was significantly reduced within the CLA group (−1.0±2.2 kg, P=0.05). CLA had no effect on RMR, physical activity or dietary intake. The rate of reported negative emotions decreased significantly with CLA, although there was no difference in any other category of adverse event. In comparison to the placebo, CLA did not affect insulin resistance, blood lipids and markers of liver function or markers of inflammation, with the exception of a significant decrease in a biomarker of endothelial dysfunction.
Conclusion:
CLA supplementation among overweight adults significantly reduced body fat over 6 months and prevented weight gain during the holiday season. Although no adverse effects were seen, additional studies should evaluate the effect of prolonged use of CLA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Notes
δ(permil)=((Runknown−Rstandard)/Rstandard)×1000, where R is the ratio of the abundance of heavy isotope to that of the light isotope.
References
Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM . Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291: 2847–2850.
Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 1998; 68: 899–917.
Pariza MW . Perspective on the safety and effectiveness of conjugated linoleic acid. Am J Clin Nutr 2004; 79: 1132S–1136S.
Wang YW, Jones PJ . Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord 2004; 28: 941–955.
Terpstra AH . Effect of conjugated linoleic acid on body composition and plasma lipids in humans: an overview of the literature. Am J Clin Nutr 2004; 79: 352–361.
Wahle KW, Heys SD, Rotondo D . Conjugated linoleic acids: are they beneficial or detrimental to health? Prog Lipid Res 2004; 43: 553–587.
Atkinson RL . Conjugated linoleic acid for altering body composition and treating obesity. Advances in conjugated linoleic acid research, volume 1. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ (eds). AOCS Press: Champagne, IL, 1999. pp. 348–353.
Berven G, Bye A, Hals O . Safety of conjugated linoleic acid (CLA) in overweight or obese volunteers. Eur J Lipid Sci Technol 2000; 102: 455–462.
Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O . Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr 2000; 130: 2943–2948.
Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 2005; 135: 778–784.
Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H et al. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr 2004; 79: 1118–1125.
Kamphuis MM, Lejeune MP, Saris WH, Westerterp-Plantenga MS . The effect of conjugated linoleic acid supplementation after weight loss on body weight regain, body composition, and resting metabolic rate in overweight subjects. Int J Obes Relat Metab Disord 2003; 27: 840–847.
Whigham LD, O'Shea M, Mohede IC, Walaski HP, Atkinson RL . Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem Toxicol 2004; 42: 1701–1709.
Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O'Neil PM, Sebring NG . A prospective study of holiday weight gain. N Engl J Med 2000; 342: 861–867.
Bartok-Olson CJ, Schoeller DA, Sullivan JC, Clark RR . The ‘B’ in the Selinger Four-Compartment body composition formula should be body mineral instead of bone mineral. Ann NY Acad Sci 2000; 904: 342–344.
Behnke A, Wilmore J . Evaluation and Regulation of Body Build and Composition. Prentice-Hall: Englewood Cliffs, NJ, 1974.
Wilmore H . A simplified method for determination of residual lung volumes. J Appl Physiol 1969; 27: 98–100.
Schoeller D, Luke A . Rapid 18O analysis of CO2 samples by continuous-flow isotope ratio mass spectrometry. J Mass Spec 1997; 32: 1332–1336.
Schoeller D . Hydrometry. In: Heymsfield S, Lohman T, Wang Z, Going S (eds). Human Body Composition. Human Kinetics: Champagne, IL, 2005. pp 35–49.
Heymsfield S, Lichtman S, Baumgartner R, Wang J, Kamen Y, Aliprantis A et al. Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr 1990; 52: 52–58.
Weir JB . New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9.
Sallis J . Seven day physical activity recall. Med Sci Sports Exerc 1997; 29: S89–S103.
Conwell LS, Trost SG, Brown WJ, Batch JA . Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 2004; 27: 314–319.
Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr 2005; 81: 74–78.
Larsen TM, Toubro S, Astrup A . Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies. J Lipid Res 2003; 44: 2234–2241.
Riserus U, Arner P, Brismar K, Vessby B . Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002; 25: 1516–1521.
Eyjolfson V, Spriet LL, Dyck DJ . Conjugated linoleic acid improves insulin sensitivity in young, sedentary humans. Med Sci Sports Exerc 2004; 36: 814–820.
Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr 2004; 80: 257–263.
Malpuech-Brugere C, Verboeket-van de Venne WP, Mensink RP, Arnal MA, Morio B, Brandolini M et al. Effects of two conjugated linoleic acid isomers on body fat mass in overweight humans. Obes Res 2004; 12: 591–598.
Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005; 135: 562–566.
Smedman A, Vessby B . Conjugated linoleic acid supplementation in humans – metabolic effects. Lipids 2001; 36: 773–781.
Schulz LO, Schoeller DA . A compilation of total daily energy expenditures and body weights in healthy adults. Am J Clin Nutr 1994; 60: 676–681.
Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003; 158: 1–13.
Acknowledgements
We thank Randall Clark and Jude Sullivan of the University of Wisconsin Hospitals and Clinics Exercise Science Laboratory for conducting body composition analysis; The University of Wisconsin General Clinical Research Center for providing in-patient support and dietary analysis; and Dr Earl Shrago for completing subject medical history and physical examinations. We acknowledge Cognis Nutrition and Health for providing test materials and sponsorship.
ACW contributed to study design, recruited subjects, completed data collection, contributed to data interpretation and wrote the manuscript. ACB contributed to study design and revised the manuscript. RNC assisted with data collection and ZZ provided assistance with statistical analysis. DAS established the study design, obtained study funding, interpreted data and revised the manuscript. None of the authors had or have any financial or personal interest in the sponsor of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watras, A., Buchholz, A., Close, R. et al. The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes 31, 481–487 (2007). https://doi.org/10.1038/sj.ijo.0803437
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803437
Keywords
This article is cited by
-
The effect of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance: a comprehensive review of putative molecular mechanisms
Nutrition & Metabolism (2023)
-
A systematic review of temporal body weight and dietary intake patterns in adults: implications on future public health nutrition interventions to promote healthy weight
European Journal of Nutrition (2022)
-
Efficacy of dietary supplements containing isolated organic compounds for weight loss: a systematic review and meta-analysis of randomised placebo-controlled trials
International Journal of Obesity (2021)
-
Dietary conjugated linoleic acid and medium-chain triglycerides for obesity management
Journal of Biosciences (2021)
-
Change in eating pattern as a contributor to energy intake and weight gain during the winter holiday period in obese adults
International Journal of Obesity (2020)