Abstract
Objective:
To study the effect of perinatal programming and overfeeding on the hypothalamic control mechanisms of food intake in adult rats.
Design:
Neonatal programming effects on body weight, food intake, central and peripheral leptin levels, hypothalamic neuropeptides, leptin receptors and central leptin responsiveness in adult rats.
Measurements:
Plasma and cerebrospinal fluid (CSF) leptin levels were analyzed using radioimmunoassay. Neuropeptide mRNA levels were analyzed using in situ hybridization. Leptin receptor mRNA levels were analyzed using reverse transcriptase-polymerase chain reaction.
Results:
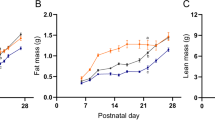
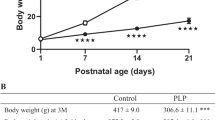
Perinatally overfed rats growing up in small litters (SL) maintain their obese and hyperleptinemic phenotype in adulthood. However, leptin levels in CSF are abnormally low considering the plasmatic hyperleptinemia. In contrast to the already reported changes in perinatally overfed juvenile rats, perinatally overfed adult rats did not show any alteration in the expression of leptin receptor isoforms and evaluated neuropeptides. Moreover, SL adult rats showed a normal sensitivity regarding the inhibitory effect of intracerebroventricular leptin administration on food intake.
Conclusion:
Perinatal overfeeding does not induce alterations in either the anorectic response to central leptin administration or expression of leptin receptors and neuropeptides in adulthood. The leptin resistance to peripheral leptin in SL adult rats may be related to impaired leptin transport across the blood–brain barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hales CN, Barker DJ . Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601.
Hales CN, Barker DJ . The thrifty phenotype hypothesis. Br Med Bull 2001; 60: 5–20.
Dorner G, Mohnike A, Honigmann G, Singer P, Padelt H . Possible significance of prenatal hyperinsulinism for postnatal development of diabetes mellitus. Endokrinologie 1973; 61: 430–432.
Vannier C, Gaillard D, Grimaldi P, Amri EZ, Djian P, Cermolacce C et al. Adipose conversion of ob17 cells and hormone-related events. Int J Obes 1985; 9 (Suppl 1): 41–53.
Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991; 303: 1019–1022.
Robinson S, Walton RJ, Clark PM, Barker DJ, Hales CN, Osmond C . The relation of fetal growth to plasma glucose in young men. Diabetologia 1992; 35: 444–446.
Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G . Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol 1992; 99: 154–158.
Ozanne SE, Hales CN . Early programming of glucose–insulin metabolism. Trends Endocrinol Metab 2002; 13: 368–373.
Ozanne SE, Lewis R, Jennings BJ, Hales CN . Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci (London) 2004; 106: 141–145.
Ozanne SE, Hales CN . Lifespan: catch-up growth and obesity in male mice. Nature 2004; 427: 411–412.
López M, Seoane LM, Tovar S, García MC, Nogueiras R, Diéguez C et al. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia 2005; 48: 140–148.
McCance RB . Food growth and time. Lancet 1962; 2: 271–272.
Oscai LB, McGarr JA . Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol 1978; 235: R141–R144.
Lucas A . Programming by early nutrition: an experimental approach. J Nutr 1998; 128 (Suppl 2): 401S–406S.
Davidowa H, Plagemann A . Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport 2000; 11: 2795–2798.
Schmidt I, Schoelch C, Ziska T, Schneider D, Simon E, Plagemann A . Interaction of genetic and environmental programming of the leptin system and of obesity disposition. Physiol Genom 2000; 3: 113–120.
Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A . The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disord 2001; 8: 1168–1174.
Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T et al. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol 1999; 11: 541–546.
Hall WG . What we know and don't know about the development of independent ingestion in rats. Appetite 1985; 6: 333–356.
López M, Seoane L, García MC, Lago F, Casanueva FF, Senarís R et al. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun 2000; 269: 41–45.
Ishizuka T, Ernsberger P, Liu S, Bedol D, Lehman TM, Koletsky RJ et al. Phenotypic consequences of a nonsense mutation in the leptin receptor gene (fak) in obese spontaneously hypertensive Koletsky rats (SHROB). J Nutr 1998; 128: 2299–2306.
Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP et al. Obesity is associated with a decreased leptin transport across the blood–brain barrier in rats. Diabetes 2000; 49: 1219–1223.
Furuhata Y, Kagaya R, Hirabayashi K, Ikeda A, Chang KT, Nishihara M et al. Development of obesity in transgenic rats with low circulating growth hormone levels: involvement of leptin resistance. Eur J Endocrinol 2000; 143: 535–541.
Grueso E, Rocha M, Puerta M . Plasma and cerebrospinal fluid leptin levels are maintained despite enhanced food intake in progesterone-treated rats. Eur J Endocrinol 2001; 144: 659–665.
Rocha M, Grueso E, Puerta M . The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. J Endocrinol 2001; 171: 349–354.
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS . Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 1999; 20: 68–100.
Meister B . Control of food intake via leptin receptors in the hypothalamus. Vit Horm 2000; 59: 265–304.
Horvath TL, Diano S, Tschop M . Brain circuits regulating energy homeostasis. Neuroscientist 2004; 10: 235–246.
López M, Seoane LM, Tovar S, Nogueiras R, Diéguez C, Señarís R . Orexin-A regulates growth hormone releasing hormone mRNA content in a nucleus specific manner and somatostatin mRNA content in a growth hormone-dependent fashion in the rat hypothalamus. Eur J Neurosci 2004; 19: 2080–2088.
Nogueiras R, Tovar S, Mitchell SE, Rayner DV, Archer ZA, Dieguez C et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 2004; 53: 2552–2558.
López M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 2006; 55: 1327–1336.
Seoane LM, López M, Tovar S, Casanueva F, Señarís R, Diéguez C . Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology 2003; 144: 544–551.
García MC, Casanueva FF, Diéguez C, Señarís R . Gestational profile of leptin messenger ribonucleic acid (mRNA) content in the placenta and adipose tissue in the rat, and regulation of the mRNA levels of the leptin receptor subtypes in the hypothalamus during pregnancy and lactation. Biol Reprod 2000; 62: 698–703.
Coll AP, Challis BG, López M, Piper S, Yeo GS, O'Rahilly S . Proopiomelanocortin-deficient mice are hypersensitive to the adverse metabolic effects of glucocorticoids. Diabetes 2005; 54: 2269–2276.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Academic Press: Sydney, 1986.
Smith MS . Lactation alters neuropeptide-Y and proopiomelanocortin gene expression in the arcuate nucleus of the rat. Endocrinology 1993; 133: 1258–1265.
Li C, Chen P, Smith MS . The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology 1998; 139: 1645–1652.
Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS . Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology 1999; 140: 2645–2650.
Hahn TM, Breininger JF, Baskin DG, Schwartz MW . Coexpression of AgRP and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1998; 1: 271–272.
Davidowa H, Li Y, Plagemann A . Altered responses to orexigenic (AGRP, MCH) and anorexigenic (alpha-MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci 2003; 18: 613–621.
Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 1996; 348: 159–161.
Flier JS . Obesity wars: molecular progress confronts an expanding epidemic. Cell 2004; 116: 337–350.
Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM . Leptin enters the brain by a saturable system independent of insulin. Peptides 1996; 17: 305–311.
Kastin AJ, Pan W . Dynamic regulation of leptin entry into brain by the blood–brain barrier. Regul Pept 2000; 92: 37–43.
Banks WA, Farrell CL . Impaired transport of leptin across the blood–brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 2003; 285: E10–E15.
Ladyman SR, Grattan DR . Region specific reduction in leptin-induced phosphorylation of STAT3 in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 2004; 145: 3704–3711.
Munzberg H, Flier JS, Bjorbaek C . Region-specific leptin resistance within the hypothalamus of diet-induced-obese mice. Endocrinology 2004; 145: 4880–4889.
Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS . Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 2004; 10: 734–738.
Levin BE, Dunn-Meynell AA, Banks WA . Obesity-prone rats have normal blood–brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 2004; 286: R143–R150.
Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997; 99: 385–390.
Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS . Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 2000; 279: E838–E845.
Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004; 304: 110–115.
Bouret SG, Draper SJ, Simerly RB . Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004; 304: 108–110.
Koistinen HA, Karonen SL, Iivanainen M, Koivisto VA . Circulating leptin has saturable transport into intrathecal space in humans. Eur J Clin Invest 1998; 28: 894–897.
Jeanrenaud B, Rohner-Jeanrenaud F . Effects of neuropeptides and leptin on nutrient partitioning: dysregulations in obesity. Annu Rev Med 2001; 52: 339–351.
Singhal A, Farooqi IS, O'Rahilly S, Cole TJ, Fewtrell M, Lucas A . Early nutrition and leptin concentrations in later life. Am J Clin Nutr 2002; 75: 993–999.
Acknowledgements
We thank Luz Casas for her excellent technical assistance and Daniel Lam (University of Cambridge, UK) for his comments and criticisms. This work has been supported by grants from Fondo de Investigaciones Sanitarias, Spanish Ministry of Health, Xunta de Galicia, DGICYT and European Union (LSHM-CT-2003-503041).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary information
Rights and permissions
About this article
Cite this article
López, M., Tovar, S., Vázquez, M. et al. Perinatal overfeeding in rats results in increased levels of plasma leptin but unchanged cerebrospinal leptin in adulthood. Int J Obes 31, 371–377 (2007). https://doi.org/10.1038/sj.ijo.0803425
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803425
Keywords
This article is cited by
-
Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21
Nature Metabolism (2022)
-
Feeding circuit development and early-life influences on future feeding behaviour
Nature Reviews Neuroscience (2018)
-
The cellular and molecular bases of leptin and ghrelin resistance in obesity
Nature Reviews Endocrinology (2017)
-
Sequential Exposure to Obesogenic Factors in Females Rats: From Physiological Changes to Lipid Metabolism in Liver and Mesenteric Adipose Tissue
Scientific Reports (2017)
-
Effects of a High-Fat Diet on Adipose Tissue CD8+ T Cells in Young vs. Adult Mice
Inflammation (2017)