Abstract
OBJECTIVE: To compare the long-term compliance and effects of two low-fat diets differing in carbohydrate to protein ratio on body composition and biomarkers of cardiovascular disease risk in obese subjects with hyperinsulinemia.
DESIGN: Outpatient, parallel, clinical intervention study of two groups of subjects randomly assigned to either a standard protein (SP; 15% protein, 55% carbohydrate) or high-protein (HP; 30% protein, 40% carbohydrate) diet, during 12 weeks of energy restriction (∼6.5 MJ/day) and 4 weeks of energy balance (∼8.3 MJ/day). Subsequently, subjects were asked to maintain the same dietary pattern for the succeeding 52 weeks with minimal professional support.
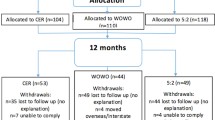
SUBJECTS: A total of 58 obese, nondietetic subjects with hyperinsulinemia (13 males/45 females, mean age 50.2 y, mean body mass index (BMI) 34.0 kg/m2, mean fasting insulin 17.8 mU/l) participated in the study.
MEASUREMENTS: Body composition, blood pressure, blood lipids, fasting glucose, insulin, CRP and sICAM-1 were measured at baseline and at weeks 16 and 68. Urinary urea/creatinine ratio was measured at baseline, week 16 and at 3 monthly intervals thereafter.
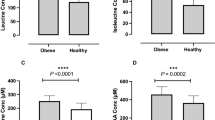
RESULTS: In total, 43 subjects completed the study with similar dropouts in each group (P=0.76). At week 68, there was net weight loss (SP −2.9±3.6%, HP −4.1±5.8%; P<0.01) due entirely to fat loss (P<0.001) with no diet effect. Both diets significantly increased HDL cholesterol concentrations (P<0.001) and decreased fasting insulin, insulin resistance, sICAM-1 and CRP levels (P<0.05). Protein intake was significantly greater in HP during the initial 16 weeks (P<0.001), but decreased in HP and increased in SP during 52-week follow-up, with no difference between groups at week 68, indicating poor long-term dietary adherence behaviour to both dietary patterns.
CONCLUSION: Without active ongoing dietary advice, adherence to dietary intervention is poor. Nonetheless, both dietary patterns achieved net weight loss and improvements in cardiovascular risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bonow RO, Eckel RH . Diet, obesity, and cardiovascular risk. N Engl J Med 2003; 348: 2057–2058.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH . The disease burden associated with overweight and obesity. JAMA 1999; 282: 1523–1529.
Flegal KM, Carroll MD, Ogden CL, Johnson CL . Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002; 288: 1723–1727.
Cameron AJ, Welborn TA, Zimmet PZ, Dunstan DW, Owen N, Salmon J, Dalton M, Jolley D, Shaw JE . Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427–432.
Visscher TL, Seidell JC . The public health impact of obesity. Annu Rev Public Health 2001; 22: 355–375.
Seidell JC . The impact of obesity on health status: some implications for health care costs. Int J Obes Relat Metab Disord 1995; 19 (Suppl 6): S13–S16.
Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW . Prevalence of attempting weight loss and strategies for controlling weight. JAMA 1999; 282: 1353–1358.
Eisenstein J, Roberts SB, Dallal G, Saltzman E . High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev 2002; 60: 189–200.
Hill JO, Peters JC . Environmental contributions to the obesity epidemic. Science 1998; 280: 1371–1374.
Westerterp KR, Verboeket-van de Venne WP, Westerterp-Plantenga MS, Velthuis-te Wierik EJ, de Graaf C, Weststrate JA . Dietary fat and body fat: an intervention study. Int J Obes Relat Metab Disord 1996; 20: 1022–1026.
Toubro S, Astrup A . Randomised comparison of diets for maintaining obese subjects' weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997; 314: 29–34.
Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA . High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord 1999; 23: 1202–1206.
Skov AR, Toubro S, Ronn B, Holm L, Astrup A . Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23: 528–536.
Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD . A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 2003; 133: 411–417.
Parker B, Noakes M, Luscombe N, Clifton P . Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425–430.
Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G, Librenti MC, Galli-Kienle M, Pontiroli AE, Pozza G . Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism 1994; 43: 1481–1487.
Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM . Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr 2003; 78: 31–39.
Wolfe BM, Giovannetti PM . Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism 1991; 40: 338–343.
Hodge A, Patterson AJ, Brown WJ, Ireland P, Giles G . The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust N Z J Public Health 2000; 24: 576–583.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L . Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol 1981; 11: 523–526.
Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A . Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord 1999; 23: 1170–1177.
Poppitt SD, McCormack D, Buffenstein R . Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav 1998; 64: 279–285.
Latner JD, Schwartz M . The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999; 33: 119–128.
Bray GA, Popkin BM . Dietary fat intake does affect obesity! Am J Clin Nutr 1998; 68: 1157–1173.
Heshka S, Greenway F, Anderson JW, Atkinson RL, Hill JO, Phinney SD, Miller-Kovach K, Xavier Pi-Sunyer F . Self-help weight loss versus a structured commercial program after 26 weeks: a randomized controlled study. Am J Med 2000; 109: 282–287.
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S . A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003; 348: 2082–2090.
Thomas PR, (ed), The Food and Nutrition Board, Institute of Medicine Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Weighing the options: criteria for evaluating weight management programs. National Academy Press: Washington, DC; 1995.
World Health Organisation. Obesity: preventing and managing the global epidemic. WHO Publications: Geneva; 1997.
Granberry MC, Fonseca VA . Insulin resistance syndrome: options for treatment. South Med J 1999; 92: 2–15.
Ross R . Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–126.
Blake GJ, Ridker PM . Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med 2002; 252: 283–294.
Hak AE, Pols HA, Stehouwer CD, Meijer J, Kiliaan AJ, Hofman A, Breteler MM, Witteman JC . Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab 2001; 86: 4398–4405.
Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J . Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998; 351: 88–92.
Ridker PM, Hennekens CH, Buring JE, Rifai N . C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342: 836–843.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
Albert MA, Danielson E, Rifai N, Ridker PM . Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286: 64–70.
Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto Jr AM, Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001; 344: 1959–1965.
Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E . Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1998; 98: 839–844.
Szapary PO, Rader DJ . Pharmacological management of high triglycerides and low high-density lipoprotein cholesterol. Curr Opin Pharmacol 2001; 1: 113–120.
Boden WE . High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High-Density Lipoprotein Intervention Trial. Am J Cardiol 2000; 86: 19L–22L.
Dattilo AM, Kris-Etherton PM . Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr 1992; 56: 320–328.
Yu-Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris-Etherton PM . Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am J Clin Nutr 1999; 69: 632–646.
Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP . Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000; 15: 2504–2512.
Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E . Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol 2002; 155: 636–644.
Brandle E, Sieberth HG, Hautmann RE . Effect of chronic dietary protein intake on the renal function in healthy subjects. Eur J Clin Nutr 1996; 50: 734–740.
Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A . Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res 2002; 10: 432–438.
Tremblay A, Doucet E, Imbeault P . Physical activity and weight maintenance. Int J Obes Relat Metab Disord 1999; 23 (Suppl 3): S50–S54.
Fogelholm M, Kukkonen-Harjula K . Does physical activity prevent weight gain—a systematic review. Obes Rev 2000; 1: 95–111.
Acknowledgements
We acknowledge Rosemary McArthur, Anne McGuffin, and Jodie Avery for assistance in performing these studies. This work was supported by a National Health and Medical Research Grant #158012 and a Dairy Research and Development Corporation Grant #CSHN100003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brinkworth, G., Noakes, M., Keogh, J. et al. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes 28, 661–670 (2004). https://doi.org/10.1038/sj.ijo.0802617
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802617
Keywords
This article is cited by
-
Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of obesity, Korean Diabetes Association, and Korean Society of Hypertension
Clinical Hypertension (2022)
-
Effect of a high-protein diet on maintenance of blood pressure levels achieved after initial weight loss: the DiOGenes randomized study
Journal of Human Hypertension (2015)
-
Prevalence of Anemia and Related Deficiencies 10 Years After Gastric Bypass—a Retrospective Study
Obesity Surgery (2015)
-
Adolescent-Onset Depression: Are Obesity and Inflammation Developmental Mechanisms or Outcomes?
Child Psychiatry & Human Development (2015)
-
Randomized controlled trial of a computer-tailored multiple health behaviour intervention in general practice: 12-month follow-up results
International Journal of Behavioral Nutrition and Physical Activity (2014)