Abstract
The point mutation at bp 8993 of human mtDNA in the ATPase 6 gene is associated with neurogenic weakness, ataxia and retinitis pigmentosa, and with subacute necrotizing encephalomyelopathy (Leigh disease) when present at high copy number. In this study we describe three new multiplex families with the ATPase 8993 mtDNA mutation and demonstrate a correlation between the percentage heteroplasmy of this mutation and the clinical phenotype. By combining this study with previous data we produce a graph of age of onset of symptoms versus percentage heteroplasmy of the mutation. Finally, we determine that ATP synthesis with NAD-linked substrates in cultured lymphoblast mitochondria from three patients with Leigh disease who had a high percentage heteroplasmy was on average 66% of the rate seen in control lymphoblast mitochondria. Similar rates are observed in lymphoblast mitochondria isolated from patients with Leigh disease due to complex I deficiency. This percentage appears to be independent of the rate of electron transport in mitochondria from patient cell lines with the mtDNA 8993 mutation.
Similar content being viewed by others
Introduction
The point mutation at 8993 of human mtDNA in the ATPase 6 gene has been associated with the human disease phenotype of neurogenic weakness, ataxia and retinitis pigmentosa (NARP) [1–3]. More recent descriptions of patients with this mutation have included subacute necrotizing encephalomyelopathy (Leigh disease), mental retardation with ataxia and retinitis pigmentosa, and Leigh disease and cardiomyopathy [3–5].
The 8993 T to G mutation is heteroplasmic in nature, such that a high copy number (75–100%) of the mutated ATPase 6 gene gives rise to disease while patients with lower copy numbers (<50%) tend to be asymptomatic [3–5]. In this study we correlate the percentage of heteroplasmy with the clinical presentation in individuals from three new families. We also give an estimate of how the 8993 mutation affects the overall efficiency of oxidative phosphorylation.
Materials and Methods
Lymphoblast cultures were established from family members and cultured in RPMI medium with 15% fetal calf serum. DNA was extracted either from whole blood [6] using Chelex resin or from lymphocytes by the methods of Old [7].
For amplification of mtDNA, 500 ng total DNA was amplified by PCR [8] using the primers 5′-CCGACTAATCACCACCCAAC-3’ (forward) and 5′-TGTCGTGCAGGTAGAGGCTT-3’ (reverse) as used by Holt et al. [1]. After being cut with restriction enzyme AvaI the product was purified, run out on a 2% agarose gel and photographed. The negative of this photograph was then used for densitometry using a PD1 1aser densitometer model 35, equipped with version 3.2 Discovery Series software. Gels with 0.1 to 10 µg DNA PCR product were prepared and photographic negatives of these gels were used to calibrate the gel system. Percentage heteroplasmy was calculated from the ratio of cut to uncut DNA after AvaI digestion [2],
ATP synthesis in lymphoblast mitochondria was measured by a modification of the method of Robinson et al. [9]. Lymphoblast mitochondria were prepared by the method of Bourgeron et al. [10] and 30 µg mitochondria were incubated at 37°C in 200 µl of 0.25 M sucrose, 2 mM MOPS pH 7.4, 1 mM EDTA, 5 mM potassium phosphate and 1 mM ADP. After 1h, 10 µl 1.6 M perchloric acid were added to stop the reaction and the solution was centrifuged to remove precipitated protein. The ATP in this solution was then measured by enzyme fluorimetric methods [11].
To compare ATP synthesis at different rates of respiration, 30 µg lymphoblast mitochondria were incubated with 5 mM pyruvate and L-malate ranging in concentration from 1 to 50 µM Incubations with ADP and phosphate were performed to assess ATP synthesis. Rates were linear at the highest and lowest L-malate concentration for 1 h. Lymphoblast mitochondria behaved much like heart mitochondria and oxidized pyruvate rapidly with a minimal priming amount of L-malate in the micromolar range (Ka50 = 3µM).
Oligomycin-sensitive ATPase activity was measured in 30-µg samples of isolated lymphocyte mitochondria incubated in a hypotonic solution containing 50 mM KCl, 20 mM TRIS HCl pH 7.4, 2 mM ATP ± 0.2 µg/ml oligomycin [12]. Inorganic phosphate produced as a result of ATP hydrolysis was measured at 10, 15 and 30 min and was determined using standard spectrophotometric methods [13].
Case Reports
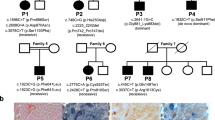
Pedigree A
The firstborn child (IV-I; fig. 1A) was born at 37.5 weeks gestation after an uncomplicated pregnancy. He was irritable and colicky as an infant, but grew and developed normally until 7 months of age when developmental testing documented motor delay. By 10 months of age, he was sitting alone and transferring objects from hand to hand. One month later, his appetite waned and he began demonstrating jackknifing spasms upon awakening; EEG monitoring confirmed hypsarrhythmia. Lactate and pyruvate were only mildly and intermittently elevated. A CT scan revealed symmetrically reduced density defects in the caudate nucleus and diffuse atrophy around the third and lateral ventricles. A muscle biopsy showed only nonspecific changes. The patient responded to adrenocorticotropic hormone (ACTH) therapy, and since the age of 3 has had a normal EEG with no evidence of seizures. However, he has had difficulty tolerating otherwise unremarkable intercurrent illnesses, becoming profoundly lethargic and taking several weeks to recover fully. His growth remains normal. He remains globally delayed but has shown no sign of developmental regression. Within the last year, an electroretinogram has shown generalized depression consistent with retinal degeneration.
Pedigrees of three families with the 8993 mtDNA T→G mutation and percentage heteroplasmy where determined. The percentage heteroplasmy mutant/total in various individuals was determined in DNA extracted from whole blood. Later measurements made on lymphoblast cultures from these blood samples gave the same percentage heteroplasmy. MR = Mental retardation; FTT = failure to thrive.
His younger brother (IV-2) was born 6 years later at 37.5 weeks gestation by cesarean section secondary to breech presentation. Mild contractures in the large joints were noted at birth; these gradually resolved with physiotherapy. Between 5 and 6 months of age gross motor delay was first suspected. He learned to roll back to front by 9 months of age, and was not yet sitting, when he demonstrated a plateauing of milestones with significantly diminished social interaction and flattening of affect, truncal ataxia, limb weakness, intermittent labored hyperventilation, and occasional drooling with difficulty swallowing. Vocalizations ceased and a time delay was noted in auditory reaction and localization. At 10 1/2 months, the patient developed infantile spasms with hypsarrhythmia on EEG. Blood lactate levels were normal although a cerebrospinal fluid level was elevated (49.4 mg/dl). Two courses of ACTH therapy were required to control the seizures. An MRI scan at 12 months of age demonstrated several high-intensity T2 signal lesions bilaterally in the basal ganglia and brainstem, as well as cerebral atrophy. An ophthalmological evaluation was normal.
The mother (III-1), maternal uncle (III-4) and his children have shown no symptoms; however, two siblings died in younger childhood with a diagnosis of Leigh disease. A maternal brother (III-2) had difficulties with feeding from birth, was always motor delayed and demonstrated little social interaction. Myoclonic jerks developed by 8 months. Death occurred at 10 months after an intercurrent illness marked by focal encephalomalacia in the basal ganglia bilaterally. A sister (III-2) followed a similar course with death occurring at 2 years of age; brain autopsy findings revealed regions of confluent foci of neovascularization, a finely vacuolated neuropil and astrocytosis in bilateral caudate, putamen and thalamic nuclei with otherwise well-preserved neurons. Cavitation was pronounced in the putamen. The brainstem was also marked by discrete areas of symmetrical vacuolization and vascular proliferation. The greatgrandmother (I-1) had late-onset retinitis pigmentosa (onset in her 50s) and ataxia (onset in her 70s).
Pedigree B
The proband (IV-1; fig. 1B) was born after an uncomplicated full-term pregnancy to his gravida I, para I, 19-year-old mother and unrelated father. Birth weight was 2.9 kg. At 3 1/2 months of age, when admitted to hospital for evaluation of a failure to thrive, he was found to have mild diffuse hypotonia with mild motor delay. Cardiac examination was normal. There was no metabolic acidosis. Cranial CT examination was normal.
At 7 1/2 months of age he developed infantile spasms with characteristic hypsarrhythmia on EEG. He had had progressive loss of milestones since 4 months of age. While in hospital, he had episodes of documented metabolic acidosis, with an arterial pH of 7.26 and Pco2 32; venous lactate was 4.5 mM and venous pyruvate was 0.27 mM. These levels remained elevated for 8 days, after which they were borderline elevated for 2 weeks (arterial lactate was 1.6 mM and venous pyruvate ranged from 0.07 to 0.13 mM). After that time, the lactate levels were normal (⩽ 1.0 mM) and the pyruvate levels were normal (⩽0.06mM). At that time, he had no purposeful movement.
Over the next month, an echocardiogram revealed a thickened left ventricle and possibly thickened right ventricle, ECG showed left ventricular dominance, cranial CT examination revealed low-density regions in the basal ganglia (globus pallidus and putamen), muscle biopsy revealed no abnormalities by light and electron microscopy, plasma amino acids and urine organic acids were normal.
During the next 5 weeks he underwent tracheostomy, remained obtunded, lost his deep-tendon reflexes, and developed nystagmus. His seizures were controlled with clonazepam. He was treated with thiamine 200 mg per day and vitamin C.
On evaluation at 14 months of age he was seizure free, hypotonic and areflexic; he was responsive only to pain; pupils were 2 mm and fixed. Cranial CT examination revealed widespread symmetric atrophy with little remaining cortex. He died at 4 years of age.
The proband’s full brother (IV-2) was said to be developmentally normal until 14 months. At that time he had three words, was pulling to a stand and walking with support. At 14 months of age he stopped using words and had a marked decrease in muscle tone, but no loss in motor skills until 28 months of age when he lost all developmental milestones during an episode of pneumonia. Over the next 4 months, he slowly regained motor skills to a 6- to 9-month level but did not regain speech. At 3 years 6 months he remained nonverbal and nonambulatory. Seizures which developed (first eye fluttering then head drop) are controlled with medication. Cranial MRI examination revealed lesions in the putamen. A muscle biopsy at 20 months revealed normal mitochondria by electron microscopy.
The proband had a maternal uncle (III-2) who was 23 years old. He was said to have mild to moderate mental retardation, and works in a sheltered workshop. He was born after an uncomplicated pregnancy, following a breech delivery, weighing 5 lb. Although he appeared normal at birth, he subsequently had delays in gross motor milestones and did not walk until he was 3 years of age. He had nonprogressive ataxia; when examined at 15 years of age he had cerebellar findings of dysmetria, dysarthria, and a wide-based gait. With intercurrent illness he would have a transient exacerbation of his ataxia. His general health was good except for seizures which started at 10 years of age with eye rolling; at 23 years of age he developed grand mal seizures. Hearing was normal and there was no history of defective dark adaptation to suggest retinal dystrophy.
The proband’s maternal grandmother (II-1) had also had a daughter who was stillborn (III-3) at term following cranial decompression for hydrocephalus, and a son (III-4) who died at 10 months of age. He had been born after an uncomplicated pregnancy following a breech delivery. Birth weight was 6 1b 6 oz. He was said to be just beginning to roll over at 10 months when he died of pneumonia. He had not had seizures. Autopsy examination indicated that he had bronchopneumonia and Schilder’s disease, although no gross or microscopic abnormalities of the brain were described. Skeletal muscle was said to be normal.
Mother and maternal grandmother were of above average intellect. Both are healthy with normal vision and dark adaptation and hearing and no abnormal cerebellar findings.
Pedigree C
The firstborn child (III-1; fig. 1C) in this family was found to be delayed in milestones at 12 months of age, and a cranial CT scan showed both ventricles to be mildly dilated. At 3 years he was able to walk, had mild ptosis, cortical atrophy and began to use three-word sentences. His blood lactate was 2.5–3.5 mM. His younger brother (III-2) had generalized hypotonia and neuromotor delay at 7 months of age and a CT scan showed mild atrophy of the cerebral hemispheres. His blood lactate was chronically elevated in the 4–6 mM range. After a number of apneic episodes and development of nystagmus he died at 1 year of age. His maternal uncle (II-2) had asthma and dysphagia while both the mother and maternal aunt are asymptomatic. A sister (I-2) of the grandmother (I-1) started to lose her sight in her 50s, being both blind and an insulin-dependent diabetic in her 80s.
Results
The percentage of mutant 8993 mtDNA in samples of blood or lymphocytes from the available family members was determined by PCR amplification of the ATPase 6 gene, cleavage with AvaI, agarose gel electrophoresis of the resultant DNA products and densitometry of gel photographs. Family A had two living representatives with Leigh disease. The older one, surviving until 7 years of age, showed 92% abnormal mtDNA while his brother with a more rapid progression of the disease had a level >95%. Their mother who was asymptomatic had 45% and the grandmother 17%. Family B had two males affected with Leigh disease, one with a rapidly progressing form; both showed mutant 8993 mtDNA at >95% levels. Their mother at 66%, grandmother at 50% and greatgrand-mother at 5% were all asymptomatic. The uncle who had mental retardation, ataxia and seizures showed 80% mutant mtDNA at 21 years. In family C, the 3-year-old child with developmental delay also showed >95% of the mutant ATPase 6 gene.
ATP synthesis in lymphoblast mitochondria from patients with a high percentage of mutant mtDNA was found to be 66% of controls with NAD-linked substrates, 57% of controls with succinate/rotenone, and 50% of controls with ascorbate/tetramethyl-p-phenylene diamine (TMPD) (fig. 2). When compared with ATP synthetic rates in mitochondria of lymphoblasts from patients with cytochrome oxidase or complex I deficiency, the rates using NAD-linked substrates were similar: 64% for complex I and 52% for cytochrome oxidase. When rates of oxidation were limited by the amount of L-malate provided with 5 mM pyruvate as substrate a wide range of ATP synthetic activity could be produced. Over a 5-fold range of activities the proportion of ATP synthetic activity in the 8993 mtDNA mutation mitochondria relative to the controls remained at around 65%, within the limits of error (fig. 3). The activity of the oligomycin-sensitive ATPase was measured in lymphoblast mitochondria from two patients with >95% of the mtDNA 8993 mutation and in two control subjects and in a maternal nephew with <5% 8993 mutation as a familial control (fig. 4). Mean activities of the two severely affected patients were 41 % of the lowest control activity and 35% of the mean control activity.
The rate of ATP synthesis by isolated lymphoblast mitochondria from patients with the mtDNA 8993 mutation. ATP synthesis was measured as described in the methods section using either 5 mM pyruvate + 5 mM L-malate, 5 mM succinate + 1 µM rotenone, or 1 mM ascorbate + 0.1 mM TMPD as substrates. Results are presented as the mean ± SEM for three controls, one patient with complex I deficiency, one patient with cytochrome oxidase deficiency, and three patients with >95% mutant 8993 mtDNA ATPase deficiency. Each patient’s lymphoblast mitochondria were tested on at least four separate occasions.
Lineweaver-Burke plot of the reciprocal of the ATP synthetic rate versus the reciprocal of the L-malate concentration. Lymphoblast mitochondria were allowed to respire with 5 mM pyruvate as substrate in the presence of increasing concentrations of L-malate. The rate of ATP synthesis was measured over a 1-hour period and the reciprocal of this rate was plotted against the reciprocal of the L-malate concentration for mitochondria from a patient with >95% 8993 mutant mtDNA (◆; 5 determinations) and five controls (□). Each point is derived from the mean ± SEM for at least four determinations.
Oligomycin-sensitive ATPase activity of two patients with Leigh disease and >95% heteroplasmy for the mt8993 mutation compared to controls. Activities of the oligomycin-sensitive ATPase were determined as described in the Materials and Methods. The values are given as the mean ± SEM for two controls (A and B), one familial control and two Leigh disease patients with >95% heteroplasmy for the 8993 mutation.
Discussion
We have presented three families in which the mtDNA 8993 mutation appears. These families, while showing many of the features described in the three families investigated and published in the literature [1, 3, 4] extend our knowledge of the disease progression and variability and emphasize some general principles involved in the ways this mutation segregates.
First of all, in all families so far described there appears to be an increase in the proportion of mutated mtDNA in subsequent generations. ‘Anticipation’ of the disease in subsequent generations has been noted for disorders due to mtDNA mutations, particularly mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) [14]. In the four families that we have reported [2, this report], plus those reported by Shoffner et al. [4], Holt et al. [1] and Ciafaloni et al. [15] there is a general tendency towards homoplasmy for the 8993 mutation in mtDNA and towards increasingly severe symptoms in successive generations. Since amplification of the mutation must take place either in the ovum germ line or in the early stages postfertilization, it is likely that some replicative advantage exists for mitochondria bearing the 8993 mutation. However, patients with this mutation do not display ragged-red fibers, so mitochondrial proliferation in muscle does not seem to occur as it does in the tRNA mutations responsible for MELAS and myoclonus epilepsy with ragged-red fibers (MERRF). With greater than 90% mutated mtDNA central nervous system involvement is variable, but in most cases there is early developmental delay. The basal ganglia are affected by 6–9 months of age with hypodense lesions visible in the thalamus, putamen, caudate nucleus or globus pallidus. Often there is dilation of the cerebral ventricles and cortical thinning due to atrophy. Progressive loss of cortical mass was observed in at least one patient after acute exacerbation of symptoms and onset of the elevation of lactate levels. In this group, seizure activity and abnormal EEG were often observable early in the disease process, before blood lactate levels became elevated. Infantile spasms with hypsarrhythmia on EEG were observed.
A milder form of the disease associated with 80% abnormal mitochondrial DNA results in delayed motor development, mental retardation, ataxia and/or preadolescent onset seizures. Retinal dystrophy can be observed in older patients with low percentage heteroplasmy. Figure 5 shows that the age of onset of disease and present age versus percentage of heteroplasmy for the 8993 mutation follows a sharp curve such that below 75%, retinal dystrophy appears to be the only observed complication, and then only in some individuals. In this region, despite the number of individuals, there was not a good correlation between percentage heteroplasmy and the presence of retinal symptoms. This might be due to variability in tissue heteroplasmy such that percentage heteroplasmy seen in lymphoblasts is not useful, or it could be that other factors, perhaps environmental in nature, have some influence on the incidence of retinitis pigmentosa in this group.
Variation in age of onset, symptoms and present age of family members carrying the 8993 mtDNA mutation as a function of percentage heteroplasmy. The percentage of mutant mtDNA 8993 is plotted as a function of age (either present or at age of onset of major symptoms). Onset age extension to present is indicated by horizontal lines where present. Data is from this work and from individuals described in Holt et al. [1] and Tatuch et al. [2]. ● = Leigh disease; ◯ = ataxia, mental retardation (± retinitis pigmentosa); □ = retinitis pigmentosa only (late onset); ■ = asymptomatic individuals.
The efficiency of oxidative phosphorylation as assessed by ATP synthesis from different patients is of the order of 50–65% when the mutation is present in high copy number. As the proportion of normal mtDNA increases so does the proportion of mitochondria with normal ATPase function. As a rough guess, 80% mtDNA heteroplasmy should produce 20% normal mitochondria and 80% with a 65% efficiency, so even the resultant 72% overall efficiency is not enough to avoid some symptoms of the disease. This may explain why apparently mild or partial decreases in other mitochondrial electron transport complexes such as complex I and complex IV result in devastating neurodegenerative disease [16, 17]. The defect in the ATPase is almost certainly a Vmax-type defect which affects efficiency to the same extent, no matter what the rate of oxidation, and which is operative in both the forward and reverse direction. The 35–40% of control acitivity observed in the hydrolysis of ATP in mitochondria is probably a true reflection of the actual activity of the F1F0 ATPase bearing the ATPase 6 mutation. This level of impairment is almost certainly operative in ATP synthesis but since we have no method of measuring this in isolation, the 50–65% of control activity we see in overall ATP synthesis due to oxidative phosphorylation probably reflects the fact that the ATPase is not the only rate-controlling enzyme in this process [18].
We showed that in cases of 8993 mtDNA mutation, ATP synthetic rates over a variety of substrate-limited oxidation rates stayed at a constant 65–67% of that in controls. This implies that despite the lowered membrane potential and pH gradient across the mitochondrial membrane, at low substrate concentrations, the impairment in ATP synthetic rates cannot be corrected by excess unused ATP synthetase capacity.
It is of interest that Hartzog and Cain [19] generated the equivalent of the 8993 mtDNA mutation in the a protein gene (equivalent of ATPase 6) of Escherichia coli by creating a Leu207 → Arg. This resultant mutated form of the F1F0 ATPase of E. coli had no activity when assessed by ATP-driven membrane vesicle acidification compared to controls. Why then is there a difference between the importance of these homologous residues in E. coli and Homo sapiens? The answer probably lies in the complexity of the F0 structure. Though these are in many ways homologous in structure in the two organisms, essential residues Glu219 and His245 in E. coli are replaced by His 168 and Glu203 in humans so that functional sites are inverted in the protein sequence [20]. Thus it is not difficult to imagine identical mutations close to these sites (Leu156 → Arg in the 8993 mutation) having quite different effects in degree in the two species.
References
Holt IJ, Harding AE, Morgan-Hughes JA: A new disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 1990;46:428–433
Tatuch Y, Christodoulou J, Feigenbaum A, Clarke JTR, Wherret J, Smith C, Rudd N, Petrova-Benedict R, Robinson BH: Heteroplasmic mtDNA mutation (T→G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am J Hum Genet 1992;50:852–858
Harding AE, Holt IJ, Sweeney MG, Brockington M, Davis MB: Prenatal diagnosis of mitochondrial DNA8993 T-G disease. Am J Hum Genet 1992;50:629–633
Shoffner JM, Fernhoff PM, Krawiecki NS, Caplan DB, Holt PJ, Wallace DC: Subacute necrotizing encephalopathy: Oxidative phosphorylation defects and the ATPase 6 mutation. Neurology 1992;42:2168–2174
Yoshinaga H, Ogino T, Ohtahara S, Sakuta R, Nonaka I, Horai S: A T to G mutation at nucleotide pair 8993 in mitochondrial DNA in a patient with Leigh syndrome. J Child Neurol 1993;8:129–133
Walsh PS, Metzger DA, Higuchi R: Rapid DNA Extraction. Biotechniques 1991; 10:506.
Old JM: Fetal DNA analysis; in Dories KE (ed): Human Genetic Diseases: A Practical Approach. Oxford, IRL, 1986.
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB: Primer dictated enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988;239:487–494
Robinson BH, Ward J, Goodyear P, Baudet A: Respiratory chain defects in the mitochondria of cultured skin fibroblasts from patients with lacticacidemia. J Clin Invest 1986;77:1422–1427
Bourgeron T, Chretien D, Rotig A, Munnich A, Rustin P: Isolation and characterization of mitochondria from human B lymphoblastoid cell lines. Biochem Biophys Res Commun 1992;186:16–23
Williamson JR, Corkey B: Assay of intermediates of the citric acid cycle and related compounds by fluorometric enzyme methods. Methods Enzymol 1969;13:434–513
Das AM, Harris DA: Defects in regulation of mitochondrial ATP synthase in cardiomyocytes from spontaneously hypertensive rats. Am J Physiol 1990;259:H1264–H1269
Bartlett GR: Phosphorous assay in column chromatography. J Biol Chem 1959;234:466–468
Ciafaloni E, Ricci E, Shanske S, et al: MELAS: Clinical features, biochemistry and molecular genetics. Ann Neurol 1992;31:391–398
Ciafaloni E, Santorelli FM, Shankse S, Deonna I, Rowlet E, et al: Maternally inherited Leigh syndrome. J Pediatr 1992;122:419–422
Glerum M, Robinson BH, Spratt C, Wilson J, Patrick D: Abnormal kinetic behaviour of cytochrome oxidase in a case of Leigh disease. Am J Hum Genet 1987;41:584–593
Robinson BH, Glerum DM, Chow W, Petrova-Benedict R, Lightowlers R, Capaldi R: The use of skin fibroblast cultures in the detection of respiratory chain defects in patients with lacticacidemia. Pediatr Res 1990;28:549–555
Letellier T, Molgat M, Mozat JP: Control of oxidative phosphorylation in rat muscle mitochondria: Implications for mitochondrial myopathies. Biochim Biophys Acta 1993;1141:58–64
Hartzog PE, Cain BD: The a Leu 207 → Arg mutation in F1F0-ATP synthase from Escherichia coli. J Biol Chem 1993;268:12250–12252.
Filiingame RH: Subunit c of F1F0 ATP synthase: Structure and role in transmembrane energy transduction. Biochim Biophys Acta 1992;1101:240–243
Acknowledgements
We thank the Canadian National Centres of Excellence Programme for supporting this work.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tatuch, Y., Pagon, R.A., Vlcek, B. et al. The 8993 mtDNA Mutation: Heteroplasmy and Clinical Presentation in Three Families. Eur J Hum Genet 2, 35–43 (1994). https://doi.org/10.1159/000472339
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1159/000472339
Key Words
This article is cited by
-
Is the spinal cord truly affected in half of the patients with Kearns-Sayre syndrome?
Neuroradiology (2020)
-
Patient-derived lymphoblastoid cell lines harboring mitochondrial DNA mutations as tool for small molecule drug discovery
BMC Research Notes (2018)
-
NARP-MILS syndrome caused by 8993 T > G mitochondrial DNA mutation: a clinical, genetic and neuropathological study
Acta Neuropathologica (2006)