Thesis |
Featured
-
-
Thesis |
 Chemists boldly go
Chemists boldly go
Michael Donnay and Michelle Francl want chemists to share the stories behind the work they do, and not be afraid to identify the heroines and heroes — and their epic adventures — that paved the way.
- Michelle Francl
- & Michael Donnay
-
Commentary |
 Teaching science through video games
Teaching science through video games
Imagine a class without lessons, tests and homework, but with missions, quests and teamwork. Video games offer an attractive educational platform because they are designed to be fun and engaging, as opposed to traditional approaches to teaching through lectures and assignments.
- Ronald A. Smaldone
- , Christina M. Thompson
- & Walter Voit
-
Thesis |
 Slow chemistry
Slow chemistry
Researchers should spend more time doing science than cataloguing every last detail about how they get it done, argues Bruce Gibb.
- Bruce Gibb
-
Thesis |
 A chemist's legacy
A chemist's legacy
Although Friedrich Stromeyer is best remembered for writing one of the founding works in plant geography — the forerunner to modern-day biogeography — his contributions to chemistry should not be underestimated, argues Malte C. Ebach.
- Malte C. Ebach
-
-
Thesis |
 Strangers to fiction
Strangers to fiction
Michelle Francl wonders if more chemists should be reading science fiction on the job.
- Michelle Francl
-
Commentary |
 One-world chemistry and systems thinking
One-world chemistry and systems thinking
The practice and overarching mission of chemistry need a major overhaul in order to be fit for purpose in the twenty-first century and beyond. The concept of 'one-world' chemistry takes a systems approach that brings together many factors, including ethics and sustainability, that are critical to the future role of chemistry.
- Stephen A. Matlin
- , Goverdhan Mehta
- & Alain Krief
-
Thesis |
 Changing chemistry by degrees
Changing chemistry by degrees
It is easy to overlook just how important temperature is when it comes to chemistry and Michelle Francl wonders if thermometers had a role in turning alchemists into chemists.
- Michelle Francl
-
Commentary |
 Another four bricks in the wall
Another four bricks in the wall
Of all the things humans can bestow names upon, new chemical elements are about the rarest. Our group of periodic table experts attempts to read the tea leaves and predict the names for elements 113, 115, 117 and 118.
- Shawn C. Burdette
- , Philip Ball
- & Brett F. Thornton
-
Article |
 Effects of correlated parameters and uncertainty in electronic-structure-based chemical kinetic modelling
Effects of correlated parameters and uncertainty in electronic-structure-based chemical kinetic modelling
Theoretical electronic-structure methods are routinely used to estimate the parameters of complex kinetic models. It is now shown that uncertainty in such model parameters is correlated and that it can be quantified. An associated sensitivity analysis method is also derived that handles complex systems with many correlated reactions.
- Jonathan E. Sutton
- , Wei Guo
- & Dionisios G. Vlachos
-
Commentary |
 Wrong but seminal
Wrong but seminal
Publishing the wrong interpretation of experimental data can result in an immediate horde of chemists feeding on the error like vultures. On rare occasions, this phenomenon can open up an entire new field of science — and the structure of ferrocene is a case in point.
- Jeffrey I. Seeman
- & Stuart Cantrill
-
Thesis |
 The tip of the iceberg
The tip of the iceberg
A day in the life of an academic, as told by Bruce C. Gibb.
- Bruce C. Gibb
-
Commentary |
 The role of chemistry in inventing a sustainable future
The role of chemistry in inventing a sustainable future
The Sustainable Development Goals adopted at a UN summit in September 2015 address many of the great challenges that our planet faces this century. Chemistry can make pivotal contributions to help realize these ambitious goals, but first it must undergo major changes in its priorities, approaches and practices.
- Stephen A. Matlin
- , Goverdhan Mehta
- & Alain Krief
-
Thesis |
 Hard-luck Scheele
Hard-luck Scheele
Carl Wilhelm Scheele had a hand in the discovery of at least six elements and contributed to the early development of chemistry in numerous other ways. Bruce Gibb looks into Scheele's story and considers why he doesn't get the credit that he deserves.
- Bruce C. Gibb
-
-
-
Thesis |
 Chemical doublespeak
Chemical doublespeak
Michelle Francl suggests that chemists should relax and not fret over ambiguous language.
- Michelle Francl
-
-
In Your Element |
 Homely holmium
Homely holmium
Brett F. Thornton and Shawn C. Burdette consider holmium's hotly contested discovery and later obscurity.
- Brett F. Thornton
- & Shawn C. Burdette
-
-
-
Thesis |
 Scents and sensibility
Scents and sensibility
It's time to wake up and smell the chemistry, argues Michelle Francl.
- Michelle Francl
-
-
-
-
Thesis |
 A molecule with a ring to it
A molecule with a ring to it
Michelle Francl wonders what makes benzene resonate with chemists.
- Michelle Francl
-
In Your Element |
 All manner of antimony
All manner of antimony
Claire Hansell surveys the uses, past and present, for antimony, including an unusual method for 'recycling' it.
- Claire Hansell
-
-
-
In Your Element |
 Gregarious gallium
Gregarious gallium
Trick cutlery and mobile phones have one peculiar element in common, as Marshall Brennan explains.
- Marshall Brennan
-
In Your Element |
 Unsporting scandium
Unsporting scandium
From Earth to the stars and back again, John Emsley surveys the uses, occurrences and mysteries of an element that is playing an increasing role in human affairs.
- John Emsley
-
Thesis |
 Faith, chemistry and extraterrestrial life
Faith, chemistry and extraterrestrial life
Bruce Gibb wonders whether our faith in chemistry — and what it can teach us about the Universe beyond our Earthly bounds — will have a role to play in the search for alien life.
- Bruce C. Gibb
-
-
Thesis |
 Seeding crystallography
Seeding crystallography
Michelle Francl wonders if the harem effect in crystallography is overrated.
- Michelle Francl
-
In Your Element |
 The colours of chromium
The colours of chromium
From rubies to Rolls-Royce, Anders Lennartson explores the colourful history of chromium and its coordination compounds.
- Anders Lennartson
-
-
Interview |
 The chemists behind the crystals
The chemists behind the crystals
Benjamin King and Dieter Schlüter, the corresponding authors of two Articles in this issue that describe single-crystal characterization of two-dimensional polymers, talk to Nature Chemistry about the background, challenges and prospects of their work.
- Claire Hansell
-
In Your Element |
 Californium gleaming
Californium gleaming
Thomas Albrecht-Schmitt explains the origin of element 98's striking green glow, and why the future for californium chemistry is just as bright.
- Thomas Albrecht-Schmitt
-
Thesis |
 An ode to the atomic weights
An ode to the atomic weights
They might not be fundamental constants of nature, but atomic weights are one of the foundations on which modern chemistry is built, explains Juris Meija.
- Juris Meija
-
News & Views |
 A big hello to halogen bonding
A big hello to halogen bonding
Halogen bonding connects a wide range of subjects — from materials science to structural biology, from computation to crystal engineering, and from synthesis to spectroscopy. The 1st International Symposium on Halogen Bonding explored the state of the art in this fast-growing field of research.
- Mate Erdelyi
-
Thesis |
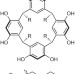 Reproducibility
Reproducibility
Bruce Gibb looks back at some examples of irreproducible reactions in his own laboratory and suggests ways in which the reproducibility of chemical reactions can be maximized.
- Bruce C. Gibb
-
In Your Element |
 Made by molybdenum
Made by molybdenum
Anders Lennartson muses on molybdenum and its essential role in catalysing reactions from the bacterial to the industrial scale.
- Anders Lennartson
-
-
Thesis |
 The write stuff
The write stuff
Michelle Francl suggests that students should be trained to write in a fashion similar to how they are taught the principles and practice of NMR spectroscopy — by providing them with a limited set of patterns and parameters.
- Michelle Francl
-
In Your Element |
 Nobelium non-believers
Nobelium non-believers
Alfred Nobel's eponymous element, nobelium, was 'first' discovered either in the 1950s or 1960s, in the USSR, Sweden or the USA. Brett F. Thornton and Shawn C. Burdette delve into the ensuing decades of internecine strife over the discovery of element 102.
- Brett F. Thornton
- & Shawn C. Burdette
-
-
In Your Element |
 Thorium lends a fiery hand
Thorium lends a fiery hand
John Arnold, Thomas L. Gianetti and Yannai Kashtan look back on thorium's chemistry, and look forward to harnessing its nuclear potential.
- John Arnold
- , Thomas L. Gianetti
- & Yannai Kashtan
-
In Your Element |
 Poisonous polonium
Poisonous polonium
Eric Ansoborlo considers the disproportionate potency of polonium compared with its relative scarcity on Earth.
- Eric Ansoborlo
-
Thesis |
 The living lab
The living lab
Bruce Gibb finds wonder in the landscape of chemistry research.
- Bruce C. Gibb

 Hedgehogs and foxes (and a bear)
Hedgehogs and foxes (and a bear) The science of silliness
The science of silliness No limit
No limit