Featured
-
-
Letter |
 Altered exocrine function can drive adipose wasting in early pancreatic cancer
Altered exocrine function can drive adipose wasting in early pancreatic cancer
Pancreatic ductal adenocarcinoma in mice induces loss of adipose tissue through altered function of the exocrine pancreas, and supplementing pancreatic enzymes attenuates the wasting of peripheral tissues induced by pancreatic cancer.
- Laura V. Danai
- , Ana Babic
- & Matthew G. Vander Heiden
-
Letter |
 Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer
Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer
The glycolytic enzyme PFKFB4 directly phosphorylates and regulates binding of the coactivator SRC-3 to ATF4 and thereby increases the transcriptional activity of this complex, leading to increased expression of metabolic genes, and enhancing tumour growth and metastasis.
- Subhamoy Dasgupta
- , Kimal Rajapakshe
- & Bert W. O’Malley
-
Letter |
 Mitochondrial translation requires folate-dependent tRNA methylation
Mitochondrial translation requires folate-dependent tRNA methylation
Mammalian mitochondria use folate-bound one-carbon units generated by the enzyme SHMT2 to methylate tRNA, and this modification is required for mitochondrial translation and thus oxidative phosphorylation.
- Raphael J. Morscher
- , Gregory S. Ducker
- & Joshua D. Rabinowitz
-
Letter |
 Clonal evolution mechanisms in NT5C2 mutant-relapsed acute lymphoblastic leukaemia
Clonal evolution mechanisms in NT5C2 mutant-relapsed acute lymphoblastic leukaemia
Mutations in the nucleotidase-encoding gene NT5C2 drive chemotherapy resistance in relapsed acute lymphoid leukaemia but the mutations also lead to a loss-of-fitness phenotype and to collateral drug sensitivity, which could be exploited for therapy.
- Gannie Tzoneva
- , Chelsea L. Dieck
- & Adolfo A. Ferrando
-
Letter |
 NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis
NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis
Cancers growing in high-oxygen environments, such as lung adenocarcinomas, select for the iron–sulfur cluster synthesizing enzyme NFS1 to support malignant proliferation and to protect from oxidative damage.
- Samantha W. Alvarez
- , Vladislav O. Sviderskiy
- & Richard Possemato
-
Article |
 Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism
Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism
The mechanism by which formaldehyde, a potent DNA and protein crosslinking agent, is generated from folate is described, with implications for the treatment of certain cancers.
- Guillermo Burgos-Barragan
- , Niek Wit
- & Ketan J. Patel
-
Letter |
 mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer
mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer
mTOR complex 1 signalling regulates polyamine metabolism and thereby promotes tumorigenesis, through regulation of the stability of a key enzyme, AMD1.
- Amaia Zabala-Letona
- , Amaia Arruabarrena-Aristorena
- & Arkaitz Carracedo
-
Review Article |
 Nutrient acquisition strategies of mammalian cells
Nutrient acquisition strategies of mammalian cells
A review of cellular strategies for nutrient sensing and acquisition, including how these strategies can be exploited by cancer cells.
- Wilhelm Palm
- & Craig B. Thompson
-
Letter |
 The metabolic function of cyclin D3–CDK6 kinase in cancer cell survival
The metabolic function of cyclin D3–CDK6 kinase in cancer cell survival
The cyclin D3–CDK6 kinase complex, which is overactive in some cancers, inhibits two key glycolysis enzymes and thereby enhances the levels of antioxidants in cells, promoting tumour cell survival.
- Haizhen Wang
- , Brandon N. Nicolay
- & Piotr Sicinski
-
Letter |
 CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells
CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells
In human cell lines with mutant KRAS and loss of LKB1, CPS1 expression correlates inversely with LKB1 expression; silencing CPS1 in these cells induces DNA damage and cell death as a result of pyrimidine depletion rather than ammonia toxicity.
- Jiyeon Kim
- , Zeping Hu
- & Ralph J. DeBerardinis
-
Letter |
 Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia
Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia
BCAT1, a cytosolic aminotransferase for branched-chain amino acids (BCAAs), is aberrantly activated and functionally required for disease progression in chronic myeloid leukaemia.
- Ayuna Hattori
- , Makoto Tsunoda
- & Takahiro Ito
-
Letter |
 Modulating the therapeutic response of tumours to dietary serine and glycine starvation
Modulating the therapeutic response of tumours to dietary serine and glycine starvation
Dependence on exogenous serine means that tumour growth is restricted in mice on a low-serine diet; this effect on tumour growth can be amplified by antagonizing the antioxidant response.
- Oliver D. K. Maddocks
- , Dimitris Athineos
- & Karen H. Vousden
-
Article |
 LACTB is a tumour suppressor that modulates lipid metabolism and cell state
LACTB is a tumour suppressor that modulates lipid metabolism and cell state
LACTB modulates mitochondrial lipid metabolism and changes the differentiation state of breast cancer cells, thereby negatively affecting the growth of various tumorigenic, but not non-tumorigenic, cells both in vitro and in vivo.
- Zuzana Keckesova
- , Joana Liu Donaher
- & Robert A. Weinberg
-
Letter |
 Metabolic gatekeeper function of B-lymphoid transcription factors
Metabolic gatekeeper function of B-lymphoid transcription factors
The B-lymphoid transcription factors PAX5 and IKZF1 restrict the supply of glucose and energy to B cells to levels that are not enough to fuel a driver-oncogene, thereby acting as tumour suppressors and sensitizing acute lymphoblastic leukaemia B cells to glucocorticoid therapy.
- Lai N. Chan
- , Zhengshan Chen
- & Markus Müschen
-
Article |
 LKB1 loss links serine metabolism to DNA methylation and tumorigenesis
LKB1 loss links serine metabolism to DNA methylation and tumorigenesis
Human tumours with mutations in LKB1 and Kras have a specific hypermetabolic state associated with increased DNA methylation, pointing to potential metabolic and epigenetic vulnerabilities of specific tumours.
- Filippos Kottakis
- , Brandon N. Nicolay
- & Nabeel Bardeesy
-
Letter |
 Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition
Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition
Accumulation of fumarate resulting from mutations in fumarate hydratase,which are associated with renal and other cancers, is shown to induce epithelial-to-mesenchymal transition—a process associated with cancer initiation.
- Marco Sciacovelli
- , Emanuel Gonçalves
- & Christian Frezza
-
Letter |
 Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion
Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion
Pancreatic adenocarcinoma cells drive autophagy in tumour microenvironment-associated stellate cells, which release alanine that is used by the cancer cells as a carbon source for a variety of metabolic processes in an otherwise nutrient-poor environment.
- Cristovão M. Sousa
- , Douglas E. Biancur
- & Alec C. Kimmelman
-
Letter |
 Reductive carboxylation supports redox homeostasis during anchorage-independent growth
Reductive carboxylation supports redox homeostasis during anchorage-independent growth
Malignant cells are able to survive and grow in detached conditions, despite the associated increase in reactive oxygen species; here a novel metabolic pathway used by cancer cells as they adapt to anchorage-independent growth is described.
- Lei Jiang
- , Alexander A. Shestov
- & Ralph J. DeBerardinis
-
Letter |
 Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities
Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities
Mutant Kras lung tumours are not a single disease but comprise two classes of tumours with distinct metabolic profiles, prognosis and therapeutic susceptibility, which can be discriminated by their relative mutant Kras allelic content.
- Emma M. Kerr
- , Edoardo Gaude
- & Carla P. Martins
-
Letter |
 Tumour-specific proline vulnerability uncovered by differential ribosome codon reading
Tumour-specific proline vulnerability uncovered by differential ribosome codon reading
Tumours can require certain amino acids for their proliferation, and the diricore method described here helps to identify such restrictive amino acids; using this method in kidney cancer tissue and breast carcinoma cells, the authors observe an association between proline deficiency and upregulation of PYCR1, an enzyme required for proline synthesis.
- Fabricio Loayza-Puch
- , Koos Rooijers
- & Reuven Agami
-
Letter |
 Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis
Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis
ASS1, a urea cycle enzyme, promotes cancer cell proliferation by facilitating pyrimidine synthesis via CAD (carbamoyl-phosphate synthase 2, aspartate transcarbamylase, and dihydroorotase complex) activation.
- Shiran Rabinovich
- , Lital Adler
- & Ayelet Erez
-
Letter |
 Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations
Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations
The first structures of the mammalian phosphofructokinase-1 tetramer are reported, for the human platelet isoform, in complex with ATP–Mg2+ and ADP.
- Bradley A. Webb
- , Farhad Forouhar
- & Diane L. Barber
-
Letter |
 SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance
SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance
Tumours are a low-oxygen environment, in this study glioblastoma cells are found to overexpress the serine hydroxymethyltransferase SHMT2; SHMT acts to reduce oxygen consumption, which confers the tumour cells with a survival advantage.
- Dohoon Kim
- , Brian P. Fiske
- & David M. Sabatini
-
Letter |
 IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo
IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo
p53 is often mutated or lost in cancer; here inactivation of ΔNp63 and ΔNp73 in the absence of p53 is shown to result in metabolic reprogramming and tumour regression via activation of IAPP (islet amyloid polypeptide or amylin), and IAPP-based anti-diabetes therapeutic strategies show potential for the treatment of p53-deficient and mutant tumours.
- Avinashnarayan Venkatanarayan
- , Payal Raulji
- & Elsa R. Flores
-
Letter |
 Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function
Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function
KRAS mutations are a driver event of pancreatic ductal adenocarcinoma; here, a subpopulation of dormant tumour cells, relying on oxidative phosphorylation for survival, is shown to be responsible for tumour relapse after treatment targeting the KRAS pathway.
- Andrea Viale
- , Piergiorgio Pettazzoni
- & Giulio F. Draetta
-
Letter |
 Fructose-1,6-bisphosphatase opposes renal carcinoma progression
Fructose-1,6-bisphosphatase opposes renal carcinoma progression
Fructose-1,6-bisphosphatase is shown to be depleted in clear cell renal cell carcinoma (ccRCC) and inhibits ccRCC progression by antagonizing glycolytic flux in renal tubular epithelial cells and by restraining cell proliferation, glycolysis, and the pentose phosphate pathway in von Hippel–Lindau-protein-deficient ccRCC cells by blocking hypoxia-inducible factor function.
- Bo Li
- , Bo Qiu
- & M. Celeste Simon
-
Letter |
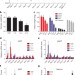 Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia
Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia
Many patients with cancer experience cachexia, a wasting disorder of adipose tissue and skeletal muscle that leads to weight loss and frailty; now, tumour-derived parathyroid-hormone-related protein has been shown to stimulate the expression of genes involved in heat production in adipose tissues and to have an important role in tissue wasting.
- Serkan Kir
- , James P. White
- & Bruce M. Spiegelman
-
Letter |
 Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer
Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer
Gain-of-function mutations in isocitrate dehydrogenase (IDH) are among the most common genetic alterations in intrahepatic cholangiocarcinoma (IHCC), a deadly cancer of the liver bile ducts; now mutant IDH is shown to block liver cell differentiation through the suppression of HNF-4α, a master regulator of hepatocyte identity and quiescence, leading to expansion of liver progenitor cells primed for progression to IHCC.
- Supriya K. Saha
- , Christine A. Parachoniak
- & Nabeel Bardeesy
-
Letter |
 The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation
The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation
A new mechanism by which acute myeloid leukaemia patients become resistant to Ara-C and a newer treatment, ribavirin, is uncovered; these drugs can be glucuronidated and thereby inactivated by members of the UDP glucuronosyltransferase family of enzymes activated through GLI1 signalling.
- Hiba Ahmad Zahreddine
- , Biljana Culjkovic-Kraljacic
- & Katherine L. B. Borden
-
Letter |
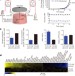 Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides
Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides
New apparatus is used to maintain proliferating cancer cells in low-glucose conditions, demonstrating that mitochondrial oxidative phosphorylation (OXPHOS) is essential for optimal proliferation in these conditions; the most sensitive cell lines are defective in OXPHOS upregulation and may therefore be sensitive to current antidiabetic drugs that inhibit OXPHOS.
- Kıvanç Birsoy
- , Richard Possemato
- & David M. Sabatini
-
Letter |
 Synthetic lethal metabolic targeting of cellular senescence in cancer therapy
Synthetic lethal metabolic targeting of cellular senescence in cancer therapy
In mice with Eµ-myc transgenic lymphomas in which therapy-induced senescence (TIS) depends on the H3K9 histone methyltransferase Suv39h1, TIS-competent lymphomas but not TIS-incompetent Suv39h1– lymphomas show increased glucose utilization and ATP production after senescence-inducing chemotherapy to cope with proteotoxic stress elicited by factors of the senescence-associated secretory phenotype (SASP); senescent cancers are selectively vulnerable to drugs that block glucose utilization or autophagy.
- Jan R. Dörr
- , Yong Yu
- & Clemens A. Schmitt
-
Letter |
 Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway
Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway
Pancreatic cancers use a novel glutamine metabolism pathway, regulated by oncogenic KRAS, to maintain redox balance; these findings add to the understanding of the mechanisms by which oncogenic alterations reprogram cellular metabolism to promote tumour growth.
- Jaekyoung Son
- , Costas A. Lyssiotis
- & Alec C. Kimmelman
-
Letter |
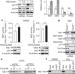 Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence
Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence
Evidence for a link between cellular senescence and metabolic regulation is provided, through the observation that p53 represses the expression of malic enzymes, thereby regulating NADPH, lipid and glutamine metabolism; in turn, this repression further activates p53, promoting cellular senescence.
- Peng Jiang
- , Wenjing Du
- & Xiaolu Yang
-
News & Views |
 Metabolism in 'the driver's seat
Metabolism in 'the driver's seat
It is increasingly accepted that metabolic changes in cancer cells can drive tumour formation. The finding that the SIRT6 protein suppresses tumour formation by regulating metabolism adds weight to this view.
- Luisa Tasselli
- & Katrin F. Chua
-
Letter |
 Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells
Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells
The authors show that p53 helps cancer cells survive serine depletion by coordinating metabolic remodelling; a diet lacking serine slowed tumour growth in mice, with p53-null tumours showing greatest sensitivity to serine starvation.
- Oliver D. K. Maddocks
- , Celia R. Berkers
- & Karen H. Vousden
-
Review Article |
 How cancer metabolism is tuned for proliferation and vulnerable to disruption
How cancer metabolism is tuned for proliferation and vulnerable to disruption
- Almut Schulze
- & Adrian L. Harris
-
News & Views |
 When more is less
When more is less
A tightly regulated enzyme balances energy production and the synthesis of macromolecules from glucose in cancer cells. Upsetting this balance by stimulating the enzyme's activity can suppress tumour growth in mice.
- Lei Jiang
- & Ralph J. DeBerardinis
-
Research Highlights |
Antitumour metabolism
-
Letter |
 AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress
AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress
A mechanism is suggested that helps tumour cells survive energy stress conditions during early stages of tumorigenesis.
- Sang-Min Jeon
- , Navdeep S. Chandel
- & Nissim Hay
-
News |
 Cancer-causing mutations yield their secrets
Cancer-causing mutations yield their secrets
Changes to metabolism disrupt cells' ability to differentiate.
- Heidi Ledford
-
News & Views |
 Sacrifice for survival
Sacrifice for survival
Cancer cells ignore oxygen availability, opting for less efficient, anaerobic ways of generating energy. The wisdom behind this choice seems to be in preventing the accumulation of reactive oxygen species, and so oxidative damage.
- Nana-Maria Grüning
- & Markus Ralser
-
Research Highlights |
Cancer: Metabolic link to breast cancer
-
-
News and Views Q&A |
 Clues from cell metabolism
Clues from cell metabolism
Interest in the abnormal metabolism exhibited by cancer cells has been reawakened by the discovery of oncogenic mutations in metabolic enzymes, and by tools that monitor metabolism in living cells. Existing and emerging therapies aim to target this abnormal metabolism in various ways.
- William G. Kaelin Jr
- & Craig B. Thompson

 Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations
Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations